��Ŀ����
����Ŀ������ͬѧ��һ�г�������һ��������װ˵����ͼ1��ʾ������ȡ��12g������Ʒ����ˮ�����Ƴ�50g��Һ��Ȼ������Һ�еμ�70g�Ȼ�����Һ�������������������μ��Ȼ�����Һ��������ϵ��ͼ2��ʾ��(�����ķ�ӦΪ��Na2CO3+CaCl2=CaCO3��+2NaCl)
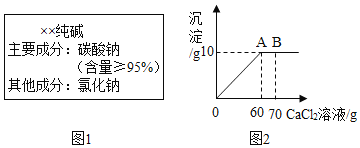
(1)ͨ�������жϴ�����Ʒ��̼���Ƶ����������Ƿ����װ˵�������__________________
(2)����A��ʱ��������Һ�����ʵ�����������( ����������һλС��) ________________
���𰸡������ 13.1%
��������
�⣺����Ʒ��̼���Ƶ�����Ϊx����Ӧ���ɵ��Ȼ��Ƶ�����Ϊy
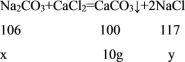
![]() ��
��![]()
��ã�x=10.6g y=11.7g
��1����Ʒ��̼���Ƶ���������Ϊ��![]()
���Դ�����Ʒ��̼���Ƶ������������װ˵���������
��2��A��ʱ��������Һ������Ϊ�Ȼ����Լ�δ��Ӧ��̼���ƣ�������Ϊ��11.7g+��12g-10.6g��=13.1g��
A��ʱ��������Һ����Һ������Ϊ��50g+60g-l0g=100g��
A��ʱ��������Һ����Һ����������Ϊ��![]() ��
��
�𣺣�1��������Ʒ��̼���Ƶ������������װ˵���������
��2��A��ʱ��������Һ�����ʵ���������Ϊ13.1%��

��ϰ��ϵ�д�
�����Ŀ