��Ŀ����
����ʮһ�ŷɴ����칬���Ž���Խӡ�̫���п�ʹ�ù���������Ϊ������������Na2O2��һ�ֵ���ɫ���壬����������ˮ�Ͷ�����̼������ѧ��Ӧ��
(1)�ڳ����£���������Na2O2���뺽��Ա������CO2��Ӧ����̼���ƺ�������������Ӧ�Ļ�ѧ����ʽΪ2Na2O2+2CO2=2Na2CO3+O2��������ͬѧ��Ϊ�÷�Ӧ����������̼������(��ѧʽΪNaHCO3)������������Ϊ����˵���Ƿ���ȷ���������Ŀ���������_______��
(2)�����£���������Ҳ����ˮ������Ӧ�����Բ���������ͬʱ�����������ƣ���д���÷�Ӧ�Ļ�ѧ����ʽΪ______��
(3)���й��ռ�վ����λ����Ա��һ��ʱ�������ų�CO2������Ϊ88g����ȫ������Щ�ų������Ҫ���Ĺ������Ƶ�����Ϊ____g��ͬʱ����ͨ��״�������������Ϊ____L(ͨ��״���µ������ܶ�Ϊ1.43g/L��������ȷ��0.1)��
 �п������п��Ծ����ϵ�д�
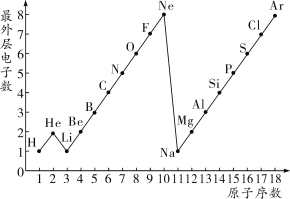
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�Na2CO3��NaHCO3�����ƵĻ�ѧ���ʣ��������ᡢijЩ�Ӧ����̼�����ȶ����� NaHCO3���ȷ֡�������
2NaHCO3 X+H2O+CO2����
X+H2O+CO2����
��1��������X�Ļ�ѧʽΪ______��
��2����ҵ����̼�������ռ�Ļ�ѧ��Ӧ����ʽΪ______��
��3��ͬѧ����̽���仯ѧ����ʵ���ʣ�����ҩƷ���з����ձ��С�С��Ϊ��Ū��ʣ�����ijɷݣ����������µ�̽����
��������룩ʣ������ǣ�I��Na2CO3����NaHCO3����______��
������ʵ�飩
���� | ���� | ���� |
��ȡ����ʣ��������Թ��У���������ˮ������ܽ�μӼ��η�̪��Һ�� | ��Һ����ɫ���ɫ�� | ʣ�����һ���У� ______ �� |
��ȡ����ʣ��������Թ��У��̶�������̨�ϣ���ּ��ȡ� | ��ɫ�����������٣� �Թ��ڱ��н϶�ˮ����֡� |
�����۷�����
�پ�����ʵ������С����Ϊ�������ȷ������ͬѧ��ΪС�����жϲ��Ͻ�����Ϊ����______Ҳ����ͬ����
��ͬѧ�����ۺ���Ϊ����Ҫȷ��ʣ�����ɷ֣�С��ֻҪ��ʵ������ڣ����������______����ͨ��______���ɵó����ۡ�
����չ��˼��
��ͬѧ�����̼��ƺ�̼������ܲ��ܻ���ת���أ�С����Ϊ���ԣ���α������Ͽ�Ƭ���н��ܵġ�ʯ�������ʯ���γɡ����ǡ�CaCO3��Ca(HCO3)2��CaCO3���Ĺ��̡���CaCO3��Ca(HCO3)2����ѧ��Ӧ����ʽΪ______��



 O2
O2 Cu2(OH)2CO3
Cu2(OH)2CO3 NaOH
NaOH NaOH
NaOH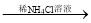 NaCl
NaCl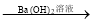 BaSO4
BaSO4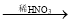 Ba(NO3)2
Ba(NO3)2