��Ŀ����
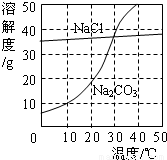
��9�֣���ͼΪ�Ȼ��ơ�̼������ˮ�е��ܽ�����ߡ�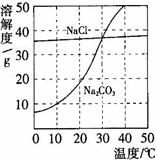
��ش��������⣺
��1����1�֣��Ȼ�����Һ�е��ܼ��� ��
��2����2�֣�10��ʱ��̼���Ƶ��ܽ��Ϊ g��
��3����2�֣�30��ʱ���Ȼ��Ƶ��ܽ�� ̼���Ƶ��ܽ�ȣ���д����������������������
��4����2�֣�10��ʱ������ֻʢ��100gˮ���ձ��У��ֱ�����Ȼ��ơ�̼�����������ʣ��������ܽ�Ϊֹ��������Һ������������������� ��Һ���ѧʽ����
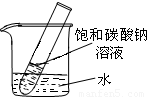
��5����2�֣�����ͼ��ʾ��20��ʱ����ʢ�б���̼������Һ��С�Թܷ���ʢˮ���ձ��У���ˮ�м���ij���ʺ��Թ����о�����������������ʿ�����________������ĸ��ţ���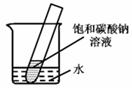
| A���������� | B����ʯ�� | C������� | D��Ũ���� |
��1��ˮ����H2O�� ��2��10 ��3��= ��4��NaCl ��5��C
����

��ϰ��ϵ�д�
 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
�����Ŀ
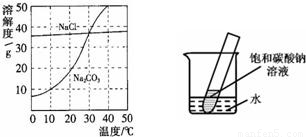
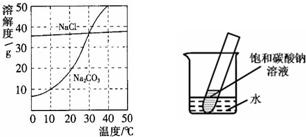 18����ͼΪ�Ȼ��ơ�̼������ˮ�е��ܽ�����ߣ���ش��������⣺
18����ͼΪ�Ȼ��ơ�̼������ˮ�е��ܽ�����ߣ���ش��������⣺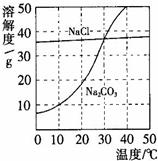 20����ͼΪ�Ȼ��ơ�̼������ˮ�е��ܽ�����ߣ�
20����ͼΪ�Ȼ��ơ�̼������ˮ�е��ܽ�����ߣ�