��Ŀ����
����A��B��C��ѡ��һ����ȷѡ�����ڱ����ڣ�Ȼ����D���ٲ���һ����������Ĵ����ں����ϡ�
(1)�����������岻��ȱ�ٵ�Ӫ�����ʡ�����ʳ���и��������ʵ���
(1)�����������岻��ȱ�ٵ�Ӫ�����ʡ�����ʳ���и��������ʵ���
[ ]
A.�߲�
B.����
C.ֲ����
D._________
B.����
C.ֲ����
D._________
(2)���л�ѧ��Ӧ�У������û���Ӧ����
[ ]
A��C+2CuO 2Cu+CO2��
2Cu+CO2��
B��C+O2 CO2
CO2
C��H2SO4+2NaOH==Na2SO4+2H2O
D��___________________________
 2Cu+CO2��
2Cu+CO2��B��C+O2
 CO2
CO2C��H2SO4+2NaOH==Na2SO4+2H2O
D��___________________________
(3)������ʵ��˵�������ڲ����˶�����
[ ]
A������ɻ�
B����������
C��ľ�ѳ���
D��_________
B����������
C��ľ�ѳ���
D��_________
(4)�����������г��������ϣ��������к���H2O��CO2����������ʡ�����ƿ��ʱ����ð�����ݡ�Ҫʹ����ð���������ݣ���ѡ�õķ�����
[ ]
A������
B����
C����ˮ
D��_______
B����
C����ˮ
D��_______
(5)���������ʯ��ˮ�����������ˮ��ƿʧȥ��ǩ����ɫҺ�壬��ѡ��
[ ]
A��ϡ����
B���Ȼ�����Һ
C����ɫʯ����Һ
D��____________
B���Ȼ�����Һ
C����ɫʯ����Һ
D��____________
(1)B������ʳƷ(����ʳƷ��)
(2)A��Fe+2HCl==FeCl2+H2��
(3)B���Ƿ���ˮ���ܽ�
(4)B������(�����)
(5)C��pH��ֽ(Na2CO3��Һ����̪��Һ��)
(2)A��Fe+2HCl==FeCl2+H2��
(3)B���Ƿ���ˮ���ܽ�
(4)B������(�����)
(5)C��pH��ֽ(Na2CO3��Һ����̪��Һ��)

��ϰ��ϵ�д�
 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д�
�����Ŀ
 CaO+CO2��
CaO+CO2��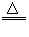 Cu+H2O
Cu+H2O