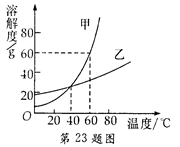
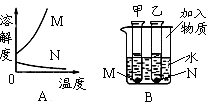
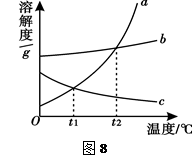
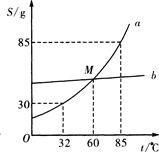
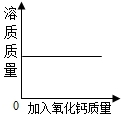
��Ŀ����
����ͼ�䷽������һƿ�����������ˮ�� ����ͼ����Ϣ�ش�
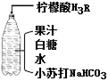
��1��С�մ��к��еĽ���Ԫ�ص������� ��
��2����ˮ�к����������ʵĻ�ѧʽ�� ��
��3����ƿ�Dz������壬˵�������ܽ���� �йأ�
��4��������ˮʱ������Ӧ�Ļ�ѧ����ʽΪ��
3NaHCO3+H3R==Na3R+ 3H2O+3X�� ����X�Ļ�ѧʽ�� ��
��5�����ǵ���Ҫ�ɷ���C12H22O11������̼��������Ԫ�ص��������� ��

��1��С�մ��к��еĽ���Ԫ�ص������� ��
��2����ˮ�к����������ʵĻ�ѧʽ�� ��
��3����ƿ�Dz������壬˵�������ܽ���� �йأ�
��4��������ˮʱ������Ӧ�Ļ�ѧ����ʽΪ��
3NaHCO3+H3R==Na3R+ 3H2O+3X�� ����X�Ļ�ѧʽ�� ��
��5�����ǵ���Ҫ�ɷ���C12H22O11������̼��������Ԫ�ص��������� ��
��1���� ��2��H2O ��3��ѹǿ ��4��CO2 ��5��72��11
��������1������С�մ�Ļ�ѧʽ����֪��������ЩԪ����ɣ��ҳ����еĽ���Ԫ�ؼ��ɣ�
��2��������ˮ�к����������ʵ���ˮ����ȷ��д�仯ѧʽ���ɣ�
��3�����������ܽ����ѹǿ�йأ����н��
��4�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû�������������ƶϣ�
��5�����ݰ���C12H22O11��̼����Ԫ�������ȵ��ڸ�ԭ�ӵ����ԭ��������ԭ�Ӹ����ıȼ��㼴�ɣ�
��𣺽⣺��1��С�մ�Ļ�ѧʽΪNaHCO3���ɴ˿�֪��С�մ�����Ԫ�ء���Ԫ�ء�̼Ԫ�غ���Ԫ����ɣ�����ֻ����Ԫ�����ڽ������ʴ�Ϊ���ƣ�
��2��������ˮ�к����������ʵ���ˮ����ѧʽΪ��H2O���ʴ�Ϊ��H2O��
��3�����������ܽ����ѹǿ�йأ��ʴ�Ϊ��ѹǿ��
��4�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû���������ڷ�Ӧ����ʽ
3NaHCO3+H3R�TNa3R+3H2O+3X�������ƶ�X�Ļ�ѧʽ�� CO2 ���ʴ�Ϊ��CO2 ��
��5��������̼����Ԫ�ص��������� ��12��12������1��22��=144��22=72��11��
�ʴ�Ϊ 144��22 �� 72��11��
��2��������ˮ�к����������ʵ���ˮ����ȷ��д�仯ѧʽ���ɣ�
��3�����������ܽ����ѹǿ�йأ����н��
��4�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû�������������ƶϣ�
��5�����ݰ���C12H22O11��̼����Ԫ�������ȵ��ڸ�ԭ�ӵ����ԭ��������ԭ�Ӹ����ıȼ��㼴�ɣ�
��𣺽⣺��1��С�մ�Ļ�ѧʽΪNaHCO3���ɴ˿�֪��С�մ�����Ԫ�ء���Ԫ�ء�̼Ԫ�غ���Ԫ����ɣ�����ֻ����Ԫ�����ڽ������ʴ�Ϊ���ƣ�
��2��������ˮ�к����������ʵ���ˮ����ѧʽΪ��H2O���ʴ�Ϊ��H2O��
��3�����������ܽ����ѹǿ�йأ��ʴ�Ϊ��ѹǿ��
��4�����������غ㶨�ɣ��ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû���������ڷ�Ӧ����ʽ
3NaHCO3+H3R�TNa3R+3H2O+3X�������ƶ�X�Ļ�ѧʽ�� CO2 ���ʴ�Ϊ��CO2 ��
��5��������̼����Ԫ�ص��������� ��12��12������1��22��=144��22=72��11��
�ʴ�Ϊ 144��22 �� 72��11��

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ