��Ŀ����
 �����еĿ�ѧ��
�����еĿ�ѧ��
��1�����ݿ����ijɷ���գ��ѧʽ����
��С������װ�п������ܱ������пɴ��һ��ʱ�䣬˵�������к���______��
�����죬С���ӿյ�������ʱ�۾��ϸ���һ��СҺ�飬˵�������к���______��
�ۺ�����װ�п����ļ���ƿ�г��ȼ�պ���ʣ�������Ҫ�ɷ�______��
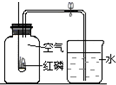
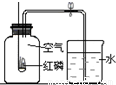
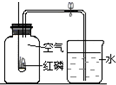
��2����֪�������������������Ϊ21%��С��ͬѧ����ͼװ�ý�����֤��ʵ����ֲ�õ������������С��1/5�������������������ֽ���Ŀ���ԭ�����ٴ�һ�㣩��______��
��3����һ�����ǽ�ڣ�����ʯ�ҽ�����Ҫ�ɷ����������ƣ���ˢ������������Ϊ��ʹǽ�ڿ�������ڷ���������һ��̿���裬�������ǽ�ڷ�������ʪ�ˣ���д��ʹ��ǽ�ڷ�������ʪ���ķ�Ӧ�Ļ�ѧ����ʽ______��
�⣺
��1�������ijɷּ����ɷֵ���������ֱ��ǣ�������78%������21%��ϡ������0.94%��������̼0.03%���������������0.03%����
�������ܹ���������С������װ�п������ܱ������пɴ��һ��ʱ�䣬˵�������к���������
��С���ӿյ�������ʱ�۾��ϸ���һ��СҺ�飬˵�������к���ˮ������
�ۺ�����װ�п����ļ���ƿ�г��ȼ�����ĵ���������ʣ�������Ҫ�ɷֵ�����
��2���ڲⶨ����������������ʵ���У����������٣�����������ȫ���ģ��������ɲ�õ������������С��1/5��
��3��ǽ�ڷ�������ʪ��ԭ���Ƕ�����̼���������Ʒ�Ӧ����ˮ���䷴Ӧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
�ʴ�Ϊ��
��1������������ˮ�������۵�����
��2�����������٣�����������ȫ���ģ�
��3��CO2+Ca��OH��2=CaCO3��+H2O��
��������1�����ݿ����ijɷּ����ɷֵ�������������жϣ�
��2�����ݿ����е������Ƿ�ȫ�����ģ�
��3��̿ȼ�����ɶ�����̼��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ��
������Ҫ��ǿ����ijɷּ����ɷֵ�����������Լ����ɷֵ���Ҫ��;��ͬʱҪ����ú��ײⶨ����������������ע�����
��1�������ijɷּ����ɷֵ���������ֱ��ǣ�������78%������21%��ϡ������0.94%��������̼0.03%���������������0.03%����
�������ܹ���������С������װ�п������ܱ������пɴ��һ��ʱ�䣬˵�������к���������
��С���ӿյ�������ʱ�۾��ϸ���һ��СҺ�飬˵�������к���ˮ������
�ۺ�����װ�п����ļ���ƿ�г��ȼ�����ĵ���������ʣ�������Ҫ�ɷֵ�����
��2���ڲⶨ����������������ʵ���У����������٣�����������ȫ���ģ��������ɲ�õ������������С��1/5��
��3��ǽ�ڷ�������ʪ��ԭ���Ƕ�����̼���������Ʒ�Ӧ����ˮ���䷴Ӧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
�ʴ�Ϊ��
��1������������ˮ�������۵�����
��2�����������٣�����������ȫ���ģ�
��3��CO2+Ca��OH��2=CaCO3��+H2O��
��������1�����ݿ����ijɷּ����ɷֵ�������������жϣ�
��2�����ݿ����е������Ƿ�ȫ�����ģ�
��3��̿ȼ�����ɶ�����̼��������̼���������Ʒ�Ӧ����̼��ƺ�ˮ��
������Ҫ��ǿ����ijɷּ����ɷֵ�����������Լ����ɷֵ���Ҫ��;��ͬʱҪ����ú��ײⶨ����������������ע�����

��ϰ��ϵ�д�
�����Ŀ
 16�������еĿ�ѧ��
16�������еĿ�ѧ��