��Ŀ����
����Ŀ����6�����������岿��ʧˮ��õ�ij����ͭ������CuSO4��xH2O������ѩͬѧΪ�о�CuSO4��xH2O���Ⱥ��������ʵ���ɣ���������ʵ�飺ȡ10.7gCuSO4��xH2O�������ʹ��ֽ⣬���Ƴ�������������¶ȵı仯��ϵͼ�������ϣ�CuSO4��x H2O����Է�������Ϊ��160+18x����ͼ��ʾ����t1��ʱ�ù�����ȫʧȥ�ᾧˮ������ѧ����ʽΪ��CuSO4��xH2O![]() CuSO4+ xH2O����
CuSO4+ xH2O����
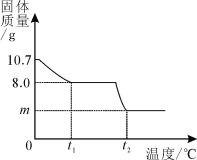
��1����3�������������ͭ������CuSO4��xH2O����x��ֵ��
��2����2��������ͭ������ͭԪ�ص����������Ƕ��٣���ȷ��0.1%��
��3����1�������µ�t2�������������Ǻ�ɫ���ʣ����ɫ������
���𰸡���1��3 ��2��29.9% ��3������ͭ
��������
���������CuSO4��xH2O ![]() CuSO4+ xH2O��
CuSO4+ xH2O��
160 18x
8.0g ��10.7-8.0��g
160��8.0 g =18x����10.7-8.0��g ��� x=3
����ͭ������ͭԪ�ص�����������=64����160+54����100%= 29.9%��
���µ�t2���������������غ㶨�ɿ����жϲ��������Ǻ�ɫ���ʣ����ɫ����������ͭ����Ϊ����ͭ����ɫ�ǰ�ɫ�Ĺ��塣

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
