��Ŀ����
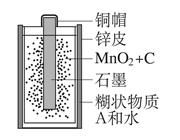
��7�֣���ͼ��ѧ������ʹ�õĽ�ͨ���ߣ����г�����������и��⣺��1���ɽ��������Ƴɵ��� ��ѡһ����������ţ������ںϳɲ��ϵ��� ��ѡһ����������ţ���

��2�����ֵĸ�Ȧ�ڳ�ʪ�Ŀ����л����⣬����ʵ��������������е�ˮ�� �����˻�����������ɵģ���ϡ����������ԭ���� ���û�ѧ����ʽ��ʾ����
��3�������������ԭ����ʳƷ��ҵ�ϻ���Ӧ�á������צ�-Fe�ۡ�����������ʳƷ���ʣ���֮Ϊ��˫�����������ܹ����տ����е�ˮ�ֺ����������û�ѧ��������ʹ��һ��ʱ���ġ�˫�������Ƿ���ȫʧЧ��д��������� ��
��4���ô���Ͳ����̥��������̥�ڿ������Ӽ�ľ��� ������ڡ�����С�ڡ������ڡ��������з���֮��ľ��룮

��2�����ֵĸ�Ȧ�ڳ�ʪ�Ŀ����л����⣬����ʵ��������������е�ˮ�� �����˻�����������ɵģ���ϡ����������ԭ���� ���û�ѧ����ʽ��ʾ����
��3�������������ԭ����ʳƷ��ҵ�ϻ���Ӧ�á������צ�-Fe�ۡ�����������ʳƷ���ʣ���֮Ϊ��˫�����������ܹ����տ����е�ˮ�ֺ����������û�ѧ��������ʹ��һ��ʱ���ġ�˫�������Ƿ���ȫʧЧ��д��������� ��
��4���ô���Ͳ����̥��������̥�ڿ������Ӽ�ľ��� ������ڡ�����С�ڡ������ڡ��������з���֮��ľ��룮
��1��C ABDE������ţ�����2��������Fe2O3+3H2SO4=Fe2��SO4��3+3H2O
��3����ϡ���ᣬ��������������ȫʧЧ����4��С�ڡ�
��3����ϡ���ᣬ��������������ȫʧЧ����4��С�ڡ�
�������ϰ���������Ͻ���������̥�����������������ںϳɲ��ϣ�������������Է�ֹ�������⣻�������Ҫ�ɷ���������������֮��ļ�����ŵ�λ�����������ӵĸ���������С��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ���ˣ����õ�����ϴ�ӡ�����������Ƶ�����Ϊa�ˣ�
���ˣ����õ�����ϴ�ӡ�����������Ƶ�����Ϊa�ˣ� ���ˣ����õ��Ĺ���ϴ�ӡ�����������Ƶ�����Ϊb�ˣ�
���ˣ����õ��Ĺ���ϴ�ӡ�����������Ƶ�����Ϊb�ˣ�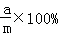 ��
��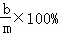 ��
��