��Ŀ����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ��װ���г��ֵ�Խ��Խ�࣬����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ����������벻����Ч����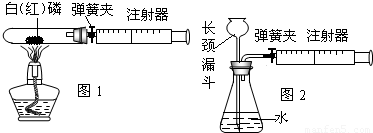
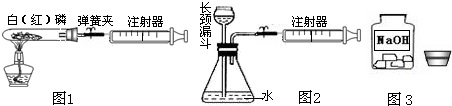
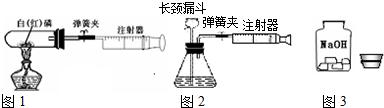
��1��ͼ1��50mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ������Ⱦ��������50mLע�������������ȴ���20mL�̶ȴ���������ȼ�����ĵ����������
�������ټ��װ�õ������ԣ���װ��ҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ���������������Ϊ______����ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�Լ______mL�̶ȴ���ȡ����ֵ����˵���������������������ԼΪ______��
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ�������������������ʱ������ܹ۲쵽______��ѡ����ţ�����˵��װ�����������ã�
A��ע��������Һ�� B��ƿ��Һ������
C������©����Һ������ D������©���¶˹ܿڲ�������
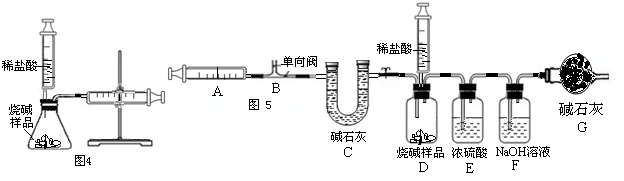
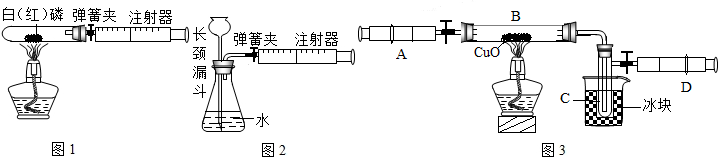
��3��ijѧ��Ϊ�˲ⶨ������Ԫ���γɵ���̬������X����ɣ�������ͼ3��ʾ��ʵ�飮����ע����A�е�����X��������װ��CuO��Bװ�ã�ʹ֮��ȫ��Ӧ���õ����½����
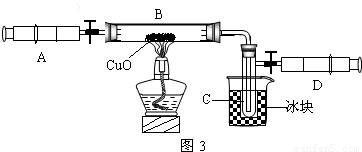
��ʵ��ǰB�ܼ�ҩƷ������Ϊ21.32g��ʵ���Ϊ21.16g��
��C�����ռ��������ʵ���õ�H2��O2����D���ռ�������N2��
��X����Ԫ�ص���������14��3���ʣ�
����C���ռ�����Һ�壬������______g��
������ʵ���п�����������______��
����B�з�Ӧ�Ļ�ѧ����ʽ��______ 3H2O+3Cu+N2
���𰸡���������1�����ð���ȼ�����Ŀ����е��������ɰ�ɫ�������������ף���ʹ�Թ������������С�����������ٵ������Ϊ�Թ��ڿ��������������������
��2��ʹ��ע�����ı�ƿ������Ķ��ٶ��ı�ƿ��ѹǿ��������������������ʱ��ƿ�����屻�����ѹǿ��С������������ӳ���©������ƿ�ڣ�����˵��װ�����������ã�
��3����ʵ��ǰB�ܼ�ҩƷ������Ϊ21.32g��ʵ���Ϊ21.16g1C�����ռ��������ʵ���ɵõ�H2��O2����D���ռ�������N2�����ǿ��Եõ�X���е���������Ԫ�أ�Ȼ�����2X����Ԫ�ص���������14��3������ȷ��X�Ļ�ѧʽ��Ȼ���������գ�
��4�����ݻ�ѧ����ʽ���м��㼴�ɣ�
����⣺��1������ʱ�¶ȴﵽ�����Ż�㣬����ȼ�գ�ȼ��ʱ���������İ��̣����ڵ��ɼм�ס������ע�������Թܵĵ��ܣ���ˣ�����ȼ��ֻ�������Թ��ڿ����е������������������������Ϊ�Թ���������֮һ��10mL�����ɼ�ʱ��ע��������������Թܣ������������Ƶ�10mL����
��2��ע��������������ʱ��ƿ�ڿ�������ѹǿ��С��װ������������ʱ���������ӳ���©������ƿ�У�����©���¶˹ܿڴ��������ݣ�
��3���������������ǿɵ�X�к��е���������Ԫ�أ���֪2X����Ԫ�ص���������14��3���������ǿɵõ���������Ԫ�ص�ԭ�Ӹ�����Ϊ1��3�����Ի�ѧʽΪNH3��
C���ռ�����ˮ������Ϊ����21.32g-21.16g��÷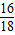 ×100%=0.18g
×100%=0.18g
��������ͭ�백����Ӧ����ͭ��ˮ�͵�������������Ϊ��ɫ������Ϊ��ɫ��
����B�з�Ӧ�Ļ�ѧ����ʽ�� 3CuO+2NH3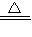 3H2O+3Cu+N2
3H2O+3Cu+N2
��4������Ҫ20%��������Һ������Ϊx��
CuO+H2SO4=CuSO4+H2O
80 98
8g 20%×x
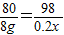
x=49g
����Ҫ���������Ϊ49g��
�ʴ�Ϊ��
��1�������������̣�10��
��2��D
��3��0.18��B�к�ɫ��ĩ��ɺ�ɫ��C������ɫҺ������
3CuO+2NH3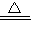 3H2O+3Cu+N2
3H2O+3Cu+N2
��4��49g
����������������ʵ�鷽�����̵�̽��������̽��ʵ��Ҫ����������������������ƵIJ����в�ͬ������ȥ˼����ȥ̽�����Ӷ��ش���Ŀ���������⣮
��2��ʹ��ע�����ı�ƿ������Ķ��ٶ��ı�ƿ��ѹǿ��������������������ʱ��ƿ�����屻�����ѹǿ��С������������ӳ���©������ƿ�ڣ�����˵��װ�����������ã�
��3����ʵ��ǰB�ܼ�ҩƷ������Ϊ21.32g��ʵ���Ϊ21.16g1C�����ռ��������ʵ���ɵõ�H2��O2����D���ռ�������N2�����ǿ��Եõ�X���е���������Ԫ�أ�Ȼ�����2X����Ԫ�ص���������14��3������ȷ��X�Ļ�ѧʽ��Ȼ���������գ�
��4�����ݻ�ѧ����ʽ���м��㼴�ɣ�
����⣺��1������ʱ�¶ȴﵽ�����Ż�㣬����ȼ�գ�ȼ��ʱ���������İ��̣����ڵ��ɼм�ס������ע�������Թܵĵ��ܣ���ˣ�����ȼ��ֻ�������Թ��ڿ����е������������������������Ϊ�Թ���������֮һ��10mL�����ɼ�ʱ��ע��������������Թܣ������������Ƶ�10mL����
��2��ע��������������ʱ��ƿ�ڿ�������ѹǿ��С��װ������������ʱ���������ӳ���©������ƿ�У�����©���¶˹ܿڴ��������ݣ�
��3���������������ǿɵ�X�к��е���������Ԫ�أ���֪2X����Ԫ�ص���������14��3���������ǿɵõ���������Ԫ�ص�ԭ�Ӹ�����Ϊ1��3�����Ի�ѧʽΪNH3��
C���ռ�����ˮ������Ϊ����21.32g-21.16g��÷
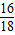 ×100%=0.18g
×100%=0.18g��������ͭ�백����Ӧ����ͭ��ˮ�͵�������������Ϊ��ɫ������Ϊ��ɫ��
����B�з�Ӧ�Ļ�ѧ����ʽ�� 3CuO+2NH3
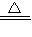 3H2O+3Cu+N2
3H2O+3Cu+N2��4������Ҫ20%��������Һ������Ϊx��
CuO+H2SO4=CuSO4+H2O
80 98
8g 20%×x
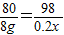
x=49g
����Ҫ���������Ϊ49g��
�ʴ�Ϊ��
��1�������������̣�10��

��2��D
��3��0.18��B�к�ɫ��ĩ��ɺ�ɫ��C������ɫҺ������
3CuO+2NH3
 3H2O+3Cu+N2
3H2O+3Cu+N2��4��49g
����������������ʵ�鷽�����̵�̽��������̽��ʵ��Ҫ����������������������ƵIJ����в�ͬ������ȥ˼����ȥ̽�����Ӷ��ش���Ŀ���������⣮

��ϰ��ϵ�д�
�����Ŀ

 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������ã�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����