��Ŀ����
��2013?��������һ�죬ijѧϰС�����Ұ�������������Ʒ���±���
��1���������в�ʳ��ʱ�����Ƿ�������ʳ�ﲻ����ƽ����ʳ��Ҫ����Ϊʳ����ȱ����
��2������Ŀ�ĵ�ͬѧ�Ƿ������˸�װʳ�κͼ��棨ֻҪ�ɷ���̼���ƣ���ƿ������ǣ�����������Ʒ
��3��Ұ����У���һͬѧ���۷��������ˣ��۷䶾Һ�����ԣ�����ͬѧ�������÷���ˮΪ��ͿĨ�ڻ��������֢״�����ᣬ������Ϊ����ˮ��
��4��Ұ��������С�ƴ������л�Ϥ��ijƷ�ƽ���ÿ100ml����Ӫ�����ı���������0.2g����Ӫ��������Ԫ����������Ϊ12.5%���ɴ˿ɼ���һƿ448ml��Ʒ�ƽ�������������Ԫ���൱�ڶ��ٿ�������������������Ԫ�������������Щ����������ͨ������ϡ���ᷴӦ�Ƶã�����Ҫ9.8%��ϡ�����������٣�
| ʳ�� | ���ס����Źǡ����Ρ����⡢���������� |
| ���� | ʳ�Ρ����桢���ǡ�ʳ�ס�ζ�������͡�ʳ���� |
| ��Ʒ | �������������롢���ӡ����� |
ˮ�����߲�
ˮ�����߲�
����2������Ŀ�ĵ�ͬѧ�Ƿ������˸�װʳ�κͼ��棨ֻҪ�ɷ���̼���ƣ���ƿ������ǣ�����������Ʒ
ʳ��
ʳ��
��������3��Ұ����У���һͬѧ���۷��������ˣ��۷䶾Һ�����ԣ�����ͬѧ�������÷���ˮΪ��ͿĨ�ڻ��������֢״�����ᣬ������Ϊ����ˮ��
��
��
�ԣ���4��Ұ��������С�ƴ������л�Ϥ��ijƷ�ƽ���ÿ100ml����Ӫ�����ı���������0.2g����Ӫ��������Ԫ����������Ϊ12.5%���ɴ˿ɼ���һƿ448ml��Ʒ�ƽ�������������Ԫ���൱�ڶ��ٿ�������������������Ԫ�������������Щ����������ͨ������ϡ���ᷴӦ�Ƶã�����Ҫ9.8%��ϡ�����������٣�
������������Ҫ��Ӫ�����������ࡢ��֬�������ʡ�ά���ء�ˮ�����Σ�
̼�����ܺ������Ե����ʷ�Ӧ���ɶ�����̼��
�����Ե������ܺ��Լ��Ե����ʷ�����Ӧ��
���ݻ�ѧʽ�ͻ�ѧ����ʽ���Խ�����ط���ļ��㣮
̼�����ܺ������Ե����ʷ�Ӧ���ɶ�����̼��
�����Ե������ܺ��Լ��Ե����ʷ�����Ӧ��
���ݻ�ѧʽ�ͻ�ѧ����ʽ���Խ�����ط���ļ��㣮
����⣺��1����������Ӫ���������֣����д����к��зḻ�����࣬���Źǡ����Ρ����⡢������ʳ���к��зḻ�ĵ����ʣ������к��зḻ�ĵ����ʺ���֬��ˮ�������ڲ���ʳ���д��ڣ���Ҫȱ��ά���أ�ˮ�����߲��к��зḻ��ά���أ�
���ˮ�����߲ˣ�
��2��ʳ�β��ܺ�ʳ��Ӧ��̼�����ܺ�ʳ��Ӧ���ɴ����ơ�ˮ�Ͷ�����̼�������������зֱ�μ�ʳ��ʱ�������ݳ��ֵ��Ǽ��棬û�������������ʳ�Σ�
���ʳ�ף�
��3���۷䶾Һ�����ԣ��ܺ��Լ��Ե����ʷ�Ӧ��Ϳ�Ϸ���ˮ��֢״�����ᣬ˵������ˮ�Լ��ԣ�
����
��4���⣺��448ml��Ʒ�ƽ�������������Ԫ���൱������ΪX��������������������Ԫ��������
0.2g���Ӽ�����Ԫ�ص�����Ϊ��0.2g��12.5%=0.025g��
448ml��������������Ԫ�ص�����Ϊ��0.025g��
=0.112g��
����������X��
��100%=0.112g��
X=0.304g��
��448ml��Ʒ�ƽ�������������Ԫ���൱��0.304g������������������Ԫ��������
�⣺����Ҫ���������ΪY��
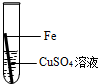
Fe+H2SO4=FeSO4+H2��
98 152
Y 0.304g
=
Y=0.196g��
����Ҫ9.8%��ϡ���������ΪZ��
����Z��9.8%=0.196g��
Z=2g��
����Ҫ9.8%��ϡ���������Ϊ2g��
���ˮ�����߲ˣ�
��2��ʳ�β��ܺ�ʳ��Ӧ��̼�����ܺ�ʳ��Ӧ���ɴ����ơ�ˮ�Ͷ�����̼�������������зֱ�μ�ʳ��ʱ�������ݳ��ֵ��Ǽ��棬û�������������ʳ�Σ�
���ʳ�ף�
��3���۷䶾Һ�����ԣ��ܺ��Լ��Ե����ʷ�Ӧ��Ϳ�Ϸ���ˮ��֢״�����ᣬ˵������ˮ�Լ��ԣ�
����
��4���⣺��448ml��Ʒ�ƽ�������������Ԫ���൱������ΪX��������������������Ԫ��������
0.2g���Ӽ�����Ԫ�ص�����Ϊ��0.2g��12.5%=0.025g��
448ml��������������Ԫ�ص�����Ϊ��0.025g��
| 448ml |
| 100ml |
����������X��
| 56 |
| 152 |
X=0.304g��
��448ml��Ʒ�ƽ�������������Ԫ���൱��0.304g������������������Ԫ��������
�⣺����Ҫ���������ΪY��
Fe+H2SO4=FeSO4+H2��
98 152
Y 0.304g
| 98 |
| 152 |
| Y |
| 0.304g |
Y=0.196g��
����Ҫ9.8%��ϡ���������ΪZ��
����Z��9.8%=0.196g��
Z=2g��
����Ҫ9.8%��ϡ���������Ϊ2g��
����������Ƚ����ף��ؼ��Ǽ���Ҫȷ�������漰����ֵ�ܷ������Բ�ע��ͻ���������������������ĿҲ�ǻᾭ�����ֵģ�

��ϰ��ϵ�д�
�����Ŀ