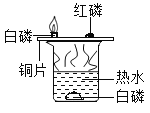
��Ŀ����
����Ŀ����ѧ���츣����Ŀ�ѧ��
��1��СС�������̺��˴����Ļ�ѧ֪ʶ����д���й����ʵĻ�ѧʽ
�ٳ���ʱҪ�ŵ�������Ϊ______________��
��ʳ���к��е���________________��
��¯�������к��еļ�____________��
�ܹܵ���Ȼ������Ҫ�ɷ�__________��
��2����ͼ����ʡ������ij�ּӸ�ʳ�ΰ�װ��ǩ�ϵIJ������֣�����ϸ�Ķ���ش��������⣺
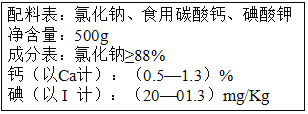
�ٰ�װ��ǩ��______Ԫ�ر���Ϊ������֮��������Ԥ����״���״�Ԫ����������______��������������������Ԫ�ء�
��̼����������ӵķ���Ϊ______��
����֪�����ӵĽṹʾ��ͼΪ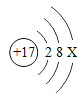 ����x����ֵΪ_______��
����x����ֵΪ_______��
���𰸡�NaCl CH3COOH NaOH CH4 I����⣩ �� Ca2+ 8
��������
��1���ٳ���ʱҪ�ŵ�����������������ָʳ�Σ���ѧ�������Ȼ��ƣ���ѧʽΪ��NaCl��
��ʳ���к��е����Ǵ��ᣬ��ѧʽΪ��CH3COOH��
��¯�������к��еļ����������ƣ���ѧʽΪ��NaOH��
����Ȼ������Ҫ�ɷ��Ǽ��飻��ѧʽΪ��CH4��
��2���ٵ��Ǻϳɼ�״�ټ��ص���ҪԪ�أ�ȱ���Ỽ��״���״���Ϊ������֮��������������Ԫ�أ�
�����ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ�����������ں�1�����ʱ��1Ҫʡ�ԣ���ʾ������������ӷ���ǰ������֣���̼����������ӵķ���Ϊ��Ca2+��
��������Ϊ�����ӣ�������=17�����������=18���ɴ˿�֪x��ֵΪ��8��

 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�����Ŀ��Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߣ����Դӱ��л������֪ʶ��ͼA(���±�)��Ԫ�����ڱ���һ����;ͼB��ͼC ��Ԫ�����ڱ�������Ԫ�ص�ԭ�ӽṹʾ��ͼ���������ͼ���ش��������⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
�ڶ� ���� | 3 Li � 7 | 4 Be �� 9 | ���� | 5 B �� 11 | �� | 7 N �� 14 | 8 O �� 16 | 9 F �� 19 | 10 Ne �� 20 |
���� ���� | 11 Na �� 23 | �� | ���� | 13 Al �� 27 | 14 Si �� 28 | �� | 16 S �� 32 | 17 Cl �� 35.5 | 18 Ar � 40 |

(1)��ͼA���ҳ�ԭ������Ϊ16��Ԫ������Ϊ_______��
(2)ͼC��ijԪ�ص�ԭ�ӽṹʾ��ͼ����ԭ���ڻ�ѧ��Ӧ����______���ӣ�
(3)ͼB��ijԪ�ص�ԭ�ӽṹʾ��ͼ����Ԫ����ͼA�е�λ����______(ѡ������ڻ��)��