��Ŀ����
����Ŀ��þ��20���Ͳŷ�չ����������������㷺Ӧ���ں��ա�������������ͨѶ���Ӻ�����ҵ�ȡ���ȤС��ͬѧ�Խ���þ����������̽����
��һ���йؽ���þ�Ļ�ѧ����̽��
��ȤС��ͬѧ���ݽ����Ļ�ѧ���ʣ�֪������þ����O2��ϡ�����CuSO4��Һ��Ӧ�������������ʵ�������֤������ʵ����þ����þ�����ѳ�ȥ����Ĥ����
��1����ȤС��ͬѧ��þ���������ھƾ��ƵĻ����ϣ��۲쵽������������˸�ŵ����ɫ�������ŵ������̻����÷�Ӧ�Ļ�ѧ����ʽ��_______________________��
��2����ȤС��ͬѧ̽��þ��ϡ����ķ�Ӧ�����۲쵽Ԥ�������⣬���۲쵽�лҰ�ɫ�������ɵġ��쳣���������ǽ���������̽����
��ʵ��1����4����ͬ�����Ũ�ȵ�ϡ�����зֱ����һ�����ȵ�ij��þ�����������£�

��ʵ�����1���ݱ�������________________��ʵ����֡��쳣��������֮һ��
��ʵ��2����4����ͬ�����Ũ�ȵ�ϡ�����зֱ����һ�����ȵ�ij��þ�����������£�
��ʵ�����2���Ա�ʵ��1��ʵ��2��______________��ʵ����֡��쳣��������֮һ��
��3����ȤС��ͬѧȡһ֧�Թܣ�����һ��þ��������һ��������ͭ��Һ���۲쵽þ���������������ݲ��������к�ɫ�������ɡ�ͬѧ�ǶԴ�ʵ�������е�������������Dz��ĵ���������ϣ�
����1������ͭ��Һ������ʱ����������ϡ���
����2����������ĩ������Сʱ�����׳�Ϊ��ɫ��
����3�����ɵĺ�ɫ���ʿ�����CuO��Cu�е�һ�ֻ����֡�
��þ��������ͭ��Һ��Ӧ��ʵ���в������������Ϊ______________��д��ѧʽ����
��ͬѧ�ǽ�һ�����ʵ��ȷ����ɫ���ʵijɷ֣������±��в�ȫʵ�鷽����
ʵ����� | ʵ������ | ���� |
����ɫ���ʹ��ˡ����ɺֳ����� | / | / |
������һ��װ��Ӳ�ʲ������У�������ͨ��CO���ȣ��������ɵ�����ͨ�����ʯ��ˮ�� | __________ | ��ɫ��ĩ�� ����CuO |
����һ�����������У���������Ϊm1���ڿ����м���һ��ʱ�����ȴ����������Ϊm2 | m1______m2��������������������� | ��ɫ��ĩ�� ����Cu |
ͨ������̽������ȤС��ͬѧȷ���˺�ɫ����ΪCu��CuO���֡�
�������й�þ������Һ�ɫ�����¡���ɵ�̽��
���������⡿þ��һ������ɫ�н�������Ľ����������õ�þ������һ��Һ�ɫ�ġ����¡������Һ�ɫ�ġ����¡���ʲô�����أ�
���������ϡ�
��1���Һ�ɫ�����¡��ijɷ��Ǽ�ʽ̼��þ���壬��ѧʽΪ��xMgCO3yMg(OH)2zH2O��
��2��Mg(OH)2����ɫ���壬������ˮ�����Ȼ�ֽ��������������
��3��MgCO3����ɫ���壬����ˮ�����Ȼ�ֽ��������������
��4����ͬ�����£�Mg(OH)2���ȷֽ���¶ȱ�MgCO3�ֽ���¶ȸ��͡�
����ɲⶨ��Ϊ��ȷ������ʽ̼��þ�������ɣ���ȤС��ͬѧȡ4.66g�þ��������ط����ǶԽ��м��ȣ�ʹ�ø��ɷ��ڲ�ͬ�¶�������ֽ⣨��������ʧȥ�ᾧˮ�����ⶨʣ�����������ֽ��¶�֮���ϵ������ͼ��ʾ����
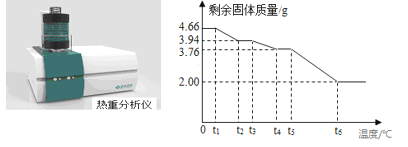
��ش��������⣺
��1�������нᾧˮ������Ϊ_______g��
��2��t4��t5���У�����Ϊ_________��д��ѧʽ����
��3��t5��t6�η�Ӧ�Ļ�ѧ����ʽΪ________��
��4������ͼ�����ݼ���xMgCO3yMg(OH)2zH2O��x��y��z =___________�������ȣ���
���𰸡� 2Mg+O2====2MgO þ�����������ȣ� ������� H2 ��ɫ��ĩ��죬����ʯ��ˮ����� m1��m2 0.72g MgO��MgCO3 MgCO3 ![]() MgO+ CO2�� x��y��z =4��1��4
MgO+ CO2�� x��y��z =4��1��4
����������һ��(1)þ��������Ӧ�Ļ�ѧ����ʽΪ��2Mg+O2![]() 2MgO��(2)��ʵ�����1���ݱ���������֪����Ϊþ����������ʣ�ർ�³������֣���ʵ�����2���Ա�ʵ��1��ʵ��2����֪������ʵ����ѡ���˲�ͬ���(3)������ͭ��Һ������ʱ����������ϡ���ᣬ����þ���������ᷴӦ�����������ڵڶ���ʵ������Ǻ�ɫ��ĩ�к���CuO��������һ��װ��Ӳ�ʲ������У�������ͨ��CO���ȣ��������ɵ�����ͨ�����ʯ��ˮ�к�ʵ������Ϊ����ɫ��ĩ��죬����ʯ��ˮ���������ɫ��ĩ�к���Cu���ڿ����м���һ��ʱ������������ͭ����Ӧ���������������ӣ�������(1)��������ʧȥ�ᾧˮ���ʾ����нᾧˮ������Ϊ��4.66g-3.94g=0.72g��(2)��ͬ�����£�Mg(OH)2���ȷֽ���¶ȱ�MgCO3�ֽ���¶ȸ��ͣ���Mg(OH)2���ȷֽ�����MgO��ˮ����t4��t5���У�����ΪMgO��MgCO3��(3)t5��t6����MgCO3�ڷֽ⣬��Ӧ�Ļ�ѧ����ʽΪ��MgCO3
2MgO��(2)��ʵ�����1���ݱ���������֪����Ϊþ����������ʣ�ർ�³������֣���ʵ�����2���Ա�ʵ��1��ʵ��2����֪������ʵ����ѡ���˲�ͬ���(3)������ͭ��Һ������ʱ����������ϡ���ᣬ����þ���������ᷴӦ�����������ڵڶ���ʵ������Ǻ�ɫ��ĩ�к���CuO��������һ��װ��Ӳ�ʲ������У�������ͨ��CO���ȣ��������ɵ�����ͨ�����ʯ��ˮ�к�ʵ������Ϊ����ɫ��ĩ��죬����ʯ��ˮ���������ɫ��ĩ�к���Cu���ڿ����м���һ��ʱ������������ͭ����Ӧ���������������ӣ�������(1)��������ʧȥ�ᾧˮ���ʾ����нᾧˮ������Ϊ��4.66g-3.94g=0.72g��(2)��ͬ�����£�Mg(OH)2���ȷֽ���¶ȱ�MgCO3�ֽ���¶ȸ��ͣ���Mg(OH)2���ȷֽ�����MgO��ˮ����t4��t5���У�����ΪMgO��MgCO3��(3)t5��t6����MgCO3�ڷֽ⣬��Ӧ�Ļ�ѧ����ʽΪ��MgCO3![]() MgO+ CO2����(4) t1��t2�μ��ٽᾧˮ������Ϊ0.72g��t3��t4����Mg(OH)2���ȷֽ�����ˮ������=3.94g-3.76g=0.18g����Mg(OH)2
MgO+ CO2����(4) t1��t2�μ��ٽᾧˮ������Ϊ0.72g��t3��t4����Mg(OH)2���ȷֽ�����ˮ������=3.94g-3.76g=0.18g����Mg(OH)2![]() H2O�������������Mg(OH)2������Ϊ0.58g��ͬ�������������MgCO3������Ϊ3.36. g����x��y��z=
H2O�������������Mg(OH)2������Ϊ0.58g��ͬ�������������MgCO3������Ϊ3.36. g����x��y��z=![]() ��
�� ![]() ��
�� ![]() =4��1��4��
=4��1��4��

����Ŀ����������ʶȿ���ͨ������pH���жϡ���������ʾ��pH���������ʶȵĹ�ϵΪ��
���� | ������ | ������ | ������ |
pH | 5.8��6.2 | 6.3��6.6 | >6.7 |
�����ϱ��жϣ�������ʹ���������Եı仯������
A.���Ա���B.���Ա�ǿC.���Բ���D.���Ա���