��Ŀ����
����Ŀ��ˮ������ͨ�����������֮һ��
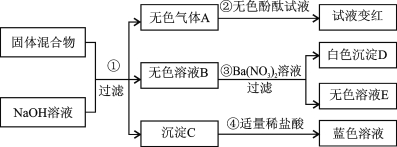
��1������������Ӳˮ�����ڽ�����Ӳˮ����ˮ��������_______________________��
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ����Ӧԭ��Ϊ:2NaCl+2H2Oͨ��2NaOH+H2��+Cl2����
��20��ʱ��NaCl���ܽ����36g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
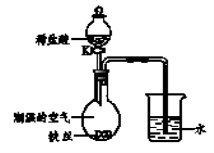
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿���ڳ�ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ��۲쵽������Һ������;��K���μ�ϡ���ᣬ�۲쵽�������� ��
���𰸡���1��Ӳˮ�к��ơ�þ���ӱ���ˮ�� ��2��2H2Oͨ��2H2��+O2��
��3���� 36��100(��9��25) �� 2NaOH+H2SO4 Na2SO4+2H2O
��4�����ܿ���Һ���½������ܿ������ݲ���
��������
�����������1������������Ӳˮ�����ڽ�����Ӳˮ����ˮ�������ǣ�Ӳˮ�к��ơ�þ���ӱ���ˮ��
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2H2Oͨ��2H2��+O2��
��3����20��ʱ��NaCl���ܽ����36g����ʾ��100gˮ�дﵽ����״̬�ܽ��NaCl������Ϊ36g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ36��100(��9��25)
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ��2NaOH+H2SO4 Na2SO4+2H2O
��4������˿���ڳ�ʪ�Ŀ����У�һ��ʱ�����˿���⣬��������ƿ�ڵ�������ʹѹǿ���ͣ�������γ�ѹǿ��ʵ�����Һ��������Ȼ���K���μ�ϡ���ᣬϡ���������ⷴӦ��ȫ����������Ӧ�������������Թ۲쵽�������ǣ����ܿ���Һ���½������ܿ������ݲ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�