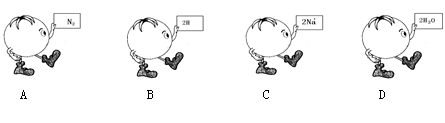
��Ŀ����
��8�֣�����H��C��N��O��Cl��Na��ѡ��ǡ����Ԫ�أ���ɷ�������Ҫ������ʣ������仯ѧʽ������Ӧ�ĺ����ϡ�
��1���ܶ���С������__________________________________��
��2����������Ҫ�ɷ�__________________________________��
��3��������ΪҺ̬��������____________________________��
��4����ѪҺ��Ѫ�쵰��ϣ�ʹ�˷����ж�������________��
��5����ʹʪ��ĺ�ɫʯ����ֽ����������________________��
��6���豣������ɫ�Լ�ƿ�о���ǿ��ʴ�Ե���____________��
��7��һ��ǿ��________________________________________��
��8����ˮ�к���������______________________________��
��1���ܶ���С������__________________________________��
��2����������Ҫ�ɷ�__________________________________��
��3��������ΪҺ̬��������____________________________��
��4����ѪҺ��Ѫ�쵰��ϣ�ʹ�˷����ж�������________��
��5����ʹʪ��ĺ�ɫʯ����ֽ����������________________��
��6���豣������ɫ�Լ�ƿ�о���ǿ��ʴ�Ե���____________��
��7��һ��ǿ��________________________________________��
��8����ˮ�к���������______________________________��
��8�֣���1��H2����2��CH4����3��H2O����4��CO����5��NH3����6��HNO3��7��NaOH����8��NaCl��
���������⿼�黯ѧ��������弰��д������ؼ��Ƿ��廯ѧ����������Ķ����Ƿ��ӡ�ԭ�ӡ����ӻ��ǻ��ϼۣ������ڻ�ѧ����ǰ������λ�ü����ʵ��ļ������������ر��������壬���ܸ������ʻ�ѧʽ����д������ȷ��д���ʵĻ�ѧʽ����������ȷ�Ľ�������Ŀ������H��C��N��O��Cl��Na��ǡ����Ԫ�أ���ɷ�������Ҫ������ʣ�
�⣺��1���ܶ���С������Ϊ��������Ϊ˫ԭ�ӵķ��ӣ��仯ѧʽΪH2��
��2����������Ҫ�ɷ�Ϊ���飬��Ϊ����л���仯ѧʽΪCH4��
��3��������ΪҺ̬��������Ϊˮ������2��1�������仯ѧʽΪH2O��
��4����ѪҺ��Ѫ�쵰��ϣ�ʹ�˷����ж�������Ϊһ����̼���仯ѧʽΪCO��
��5����ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ���Ե����壬��Ϊ�������仯ѧʽΪNH3��
��6���豣������ɫ�Լ�ƿ�о���ǿ��ʴ�Ե���Ϊ���ᣬ�仯ѧʽΪHNO3��
��7��һ��ǿ��Ϊ�������ƣ��仯ѧʽ��д����֪��Ԫ�ػ��ϼ�+1�ۣ�������-1�ۣ��ȱ�ע���ϼۣ����û��ϼ���ֵ���淨��д��ѧʽ�����仯ѧʽΪNaOH��
��8����ˮ�к���������Ϊ�Ȼ��ƣ��仯ѧʽΪNaCl��
�ʴ�Ϊ����1��H2����2��CH4����3��H2O����4��CO����5��NH3����6��HNO3��7��NaOH����8��NaCl��
������������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ȫ�棬ע�ػ�������Ŀ�ѶȽ��ף�
�⣺��1���ܶ���С������Ϊ��������Ϊ˫ԭ�ӵķ��ӣ��仯ѧʽΪH2��
��2����������Ҫ�ɷ�Ϊ���飬��Ϊ����л���仯ѧʽΪCH4��
��3��������ΪҺ̬��������Ϊˮ������2��1�������仯ѧʽΪH2O��
��4����ѪҺ��Ѫ�쵰��ϣ�ʹ�˷����ж�������Ϊһ����̼���仯ѧʽΪCO��
��5����ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ���Ե����壬��Ϊ�������仯ѧʽΪNH3��
��6���豣������ɫ�Լ�ƿ�о���ǿ��ʴ�Ե���Ϊ���ᣬ�仯ѧʽΪHNO3��
��7��һ��ǿ��Ϊ�������ƣ��仯ѧʽ��д����֪��Ԫ�ػ��ϼ�+1�ۣ�������-1�ۣ��ȱ�ע���ϼۣ����û��ϼ���ֵ���淨��д��ѧʽ�����仯ѧʽΪNaOH��
��8����ˮ�к���������Ϊ�Ȼ��ƣ��仯ѧʽΪNaCl��
�ʴ�Ϊ����1��H2����2��CH4����3��H2O����4��CO����5��NH3����6��HNO3��7��NaOH����8��NaCl��
������������Ҫ����ѧ���Ի�ѧ�������д��������������Ŀ��ƼȰ����Ի�ѧ����������˽⣬�ֿ�����ѧ���Ի�ѧ���ŵ���д������ȫ�棬ע�ػ�������Ŀ�ѶȽ��ף�

��ϰ��ϵ�д�
�����Ŀ