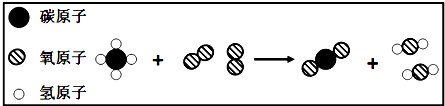
��Ŀ����
 ij��˾�������Ĵ����Ʒ�о����ֻ�����Ȼ������ʣ�Ϊ�ⶨ��Ʒ��̼���Ƶ�����������20��ʱ����ȡ�ò�Ʒ��Ʒ26.5g�����뵽ʢ��һ������ϡ������ձ��У�̼������ϡ����ǡ����ȫ��Ӧ��������ȫ�ݳ����õ�������NaCl��Һ����Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ��ͼ��ʾ��
ij��˾�������Ĵ����Ʒ�о����ֻ�����Ȼ������ʣ�Ϊ�ⶨ��Ʒ��̼���Ƶ�����������20��ʱ����ȡ�ò�Ʒ��Ʒ26.5g�����뵽ʢ��һ������ϡ������ձ��У�̼������ϡ����ǡ����ȫ��Ӧ��������ȫ�ݳ����õ�������NaCl��Һ����Ӧ�����þ�����������ձ��ڻ�����������m���뷴Ӧʱ�䣨t����ϵ��ͼ��ʾ����1���ô�����Ʒ��Na2CO3����������
��2�����õ�ϡ��������ʵ�����������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������Ӧǰ��������Ϊ��Ӧ���ɶ�����̼�����������ݶ�����̼���������Լ���̼���Ƶ�������ϡ�������Ȼ������������һ�����Լ���ô�����Ʒ��Na2CO3���������������õ�ϡ��������ʵ�����������
����⣺��1����̼���Ƶ�����Ϊx���Ȼ��������Ϊy��
��Ӧ���ɶ�����̼������Ϊ��172.5g-163.7g=8.8g��
Na2CO3+2HCl�T2NaCl+H2O+CO2����
106 73 44
x y 8.8g
=
=
��
x=21.2g��y=14.6g��
�ô�����Ʒ��Na2CO3����������Ϊ��
��100%=80%��
�𣺸ô�����Ʒ��Na2CO3����������Ϊ80%��
��2�����õ�ϡ��������ʵ���������Ϊ��
��100%=10%��
�����õ�ϡ��������ʵ���������Ϊ10%��
��Ӧ���ɶ�����̼������Ϊ��172.5g-163.7g=8.8g��
Na2CO3+2HCl�T2NaCl+H2O+CO2����
106 73 44
x y 8.8g
| 106 |
| x |
| 73 |
| y |
| 44 |
| 8.8g |
x=21.2g��y=14.6g��
�ô�����Ʒ��Na2CO3����������Ϊ��
| 21.2g |
| 26.5g |
�𣺸ô�����Ʒ��Na2CO3����������Ϊ80%��
��2�����õ�ϡ��������ʵ���������Ϊ��
| 14.6g |
| 172.5g-26.5g |
�����õ�ϡ��������ʵ���������Ϊ10%��
������������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
�����Ŀ
20��ʱ�Ȼ��Ƶ��ܽ��Ϊ36g����20��ʱ�Ȼ��Ƶı�����Һ�����ʵ���������Ϊ��������
| A��36% | B��26.5% |
| C��26.4% | D��25.6% |
��PH=1����Һ�У����Դ���������������ǣ�������
| A��K+��Na+��CH3COO-��Cl- |
| B��Al3+��Mg2+��SO42-��Cl- |
| C��Ba2+��Na+��C032-��NO3- |
| D��K+��Na+��SO42-��CO32- |
�����ǻ�ѧѧϰ�г��õ�ѧϰ������������С������ѧ��֪ʶ���й��ɣ������д����һ���ǣ�������
| A�������������ʯ��ʯ��Ũ���ᡢ��ʯ�� |
| B�������ĺϽ𣺲���֡����������� |
| C������Ӫ�����ʣ������ʡ����ࡢ��֬ |
| D�������IJ��ϣ��������ϡ������β��ϡ��л��߷��Ӳ��� |
�������ʵ������У����ڻ�ѧ���ʵ��ǣ�������
| A����������� |
| B������ʯ����ϡ���ᷴӦ |
| C���Ȼ����ǰ�ɫ���� |
| D������ȼ�ղ���������̼��ˮ |

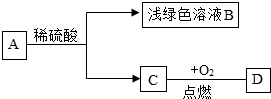 ��֪��A��B��C��D��������֮�������ͼת����ϵ������C�ǵ��ʣ�D����õ��ܼ����Իش�
��֪��A��B��C��D��������֮�������ͼת����ϵ������C�ǵ��ʣ�D����õ��ܼ����Իش�