题目内容
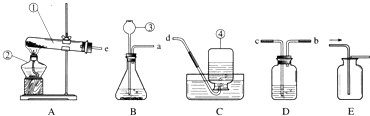
根据装置图回答问题.
(1)图中标示①仪器的名称是 _________ .
(2)实验室中既可以用来制取O2,又可以用来制取H2和CO2的发生装置是 ___(填字母代号).若用该装置制取氧气,其反应的化学方程式是 _________ .
(3)若将装置B和E连接制取氧气,停止加热时的操作是 ____ _____ (填字母代号).
_____ (填字母代号).
a、先移出导管,再熄灭酒精灯 b、先熄灭酒精灯,再移出导管
(4)若用F装置收集一瓶干燥的某气体,则该气体可能是 _________ (填字母代号).
a、HCl b、CO2 c、O2 d、NH3.
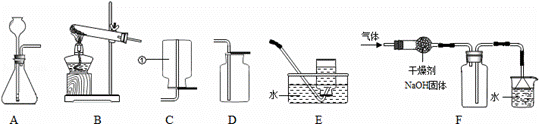
(1)图中标示①仪器的名称是 集气瓶 .
(2)实验室中既可以用来制取O2,又可以用来制取H2和CO2的发生装置是 A (填字母代号).若用该装置制取氧气,其反 应的
应的 化学方程式是 2H2O2
化学方程式是 2H2O2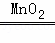 2H2O+O2↑ .
2H2O+O2↑ .
(3)若将装置B和E连接制取氧气,停止加热时的操作是 a (填字母代号).
a、先移出导管,再熄灭酒精灯 b、先熄灭酒精灯,再移出导管
(4)若用F装置收集一瓶干燥的某气体,则该气体可能是 d (填字母代号).
a、HCl b、CO2 c、O2 d、NH3.

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目