��Ŀ����
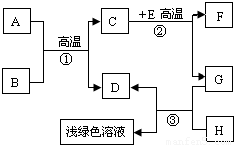
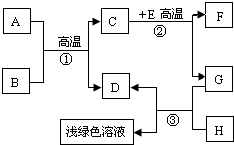
 A-H�dz��л�ѧѧϰ�г��������ʣ�����A����Ȼ������Ҫ�ɷ֣�B��������ܼ���D��G�ǵ��ʣ�C��D��F�������壮����֮���ת������ͼ��ʾ��
A-H�dz��л�ѧѧϰ�г��������ʣ�����A����Ȼ������Ҫ�ɷ֣�B��������ܼ���D��G�ǵ��ʣ�C��D��F�������壮����֮���ת������ͼ��ʾ���ش��������⣺
��1��д���������ʵĻ�ѧʽ��A
��2����Ӧ�ٺ͢ڶ����ڸ��������½��У�������������Ӧ�ڷ�Ӧʱ
��3��д��һ�����Ϸ�Ӧ�۵Ļ�ѧ����ʽ
��4����E�ʺ���ɫ��д����Ӧ�ڵĻ�ѧ����ʽΪ
��5��ʵ������ȡD�����ϡ���������ϡ�����ԭ����
������A����Ȼ������Ҫ�ɷֿ���֪��AΪ���飬B��������ܼ�����֪��BΪˮ�������ˮ�ڸ��µ�ʱ��Ӧ������һ����̼����������D�ǵ��ʣ�����DΪ��������CΪһ����̼��EΪ����ɫ�������֪��EΪ����������ôF ���Ƕ�����̼��GΪ��������HΪ���ᣬ���˸����ʼ�����ϣ����Ծݴ˴��⣮
����⣺��1��A����Ȼ������Ҫ�ɷֿ���֪��AΪ���飬B��������ܼ�����֪��BΪˮ���ʱ����Ϊ��CH4 H2O
��2��һ����Ӧ�Ƿ����Ȳ��Ǹ��ݷ�Ӧ�������жϵģ����Է�Ӧ�ٺ͢ڶ����ڸ��������½��еģ���Ҳ�����Ƿ��ȷ�Ӧ���ʱ����Ϊ����һ��
��3��dz��ɫ��Һ�������Ȼ������������������ķ�Ӧ�����������Ǽ���HΪ���ᣬ��Ӧ�ķ���ʽΪ��Fe+2HCl�TFeCl2 +H2��
��4����E�ʺ���ɫ����˵��EΪ���������ʷ�Ӧ�ڵĻ�ѧ����ʽΪ��Fe2O3+3CO
2Fe+3CO2
��5����������лӷ��ԣ���������ȡ����ʱͨ����ϡ���������ϡ���ᣬ�ʱ����Ϊ��������лӷ��ԣ���ȡ�������к����Ȼ��⣮
�ʱ����Ϊ����1��CH4 H2O
��2����һ��
��3��Fe+2HCl�TFeCl2 +H2��
��4��Fe2O3+3CO
2Fe+3CO2
��5��������лӷ��ԣ���ȡ�������к����Ȼ���
��2��һ����Ӧ�Ƿ����Ȳ��Ǹ��ݷ�Ӧ�������жϵģ����Է�Ӧ�ٺ͢ڶ����ڸ��������½��еģ���Ҳ�����Ƿ��ȷ�Ӧ���ʱ����Ϊ����һ��
��3��dz��ɫ��Һ�������Ȼ������������������ķ�Ӧ�����������Ǽ���HΪ���ᣬ��Ӧ�ķ���ʽΪ��Fe+2HCl�TFeCl2 +H2��
��4����E�ʺ���ɫ����˵��EΪ���������ʷ�Ӧ�ڵĻ�ѧ����ʽΪ��Fe2O3+3CO
| ||
��5����������лӷ��ԣ���������ȡ����ʱͨ����ϡ���������ϡ���ᣬ�ʱ����Ϊ��������лӷ��ԣ���ȡ�������к����Ȼ��⣮
�ʱ����Ϊ����1��CH4 H2O
��2����һ��
��3��Fe+2HCl�TFeCl2 +H2��
��4��Fe2O3+3CO
| ||
��5��������лӷ��ԣ���ȡ�������к����Ȼ���
����������Ϊ�ƶ��⣬��������Ŀ�ؼ��Ǵ�����и�����ѧ֪ʶ�ҳ�����-----ͻ�ƿڣ�Ȼ��˳����������������֮��ķ�Ӧ�����жϳ������ʣ���ס��ѧ����ʽ��Fe+2HCl�TFeCl2 +H2�� Fe2O3+3CO
2Fe+3CO2
| ||

��ϰ��ϵ�д�
 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
�����Ŀ
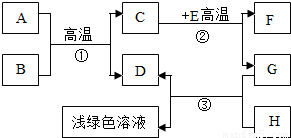
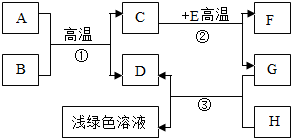
 A-H�dz��л�ѧѧϰ�г��������ʣ�����A����Ȼ������Ҫ�ɷ֣�B��������ܼ���D��G�ǵ��ʣ�C��D��F�������壮����֮���ת����ͼ��ʾ���ش��������⣺
A-H�dz��л�ѧѧϰ�г��������ʣ�����A����Ȼ������Ҫ�ɷ֣�B��������ܼ���D��G�ǵ��ʣ�C��D��F�������壮����֮���ת����ͼ��ʾ���ش��������⣺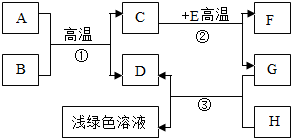 A-H�dz��л�ѧѧϰ�г��������ʣ�����A����Ȼ������Ҫ�ɷ֣�B��������ܼ���D��G�ǵ��ʣ�C��D��F�������壮����֮���ת������ͼ��ʾ��
A-H�dz��л�ѧѧϰ�г��������ʣ�����A����Ȼ������Ҫ�ɷ֣�B��������ܼ���D��G�ǵ��ʣ�C��D��F�������壮����֮���ת������ͼ��ʾ��