��Ŀ����
��2012?�ݳ���ģ�⣩�±���NaOH��Ca��OH��2���ܽ�����ݣ���ش���������
��1���ӱ������ݿ��Ի�õ���Ϣ��
��2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������
��3������20��ʱ��Ca��OH��2�ı�����Һ������Һ���������м���һ������CaO��õ�����Һ������Һ������ʱ��Һ����������������
��4��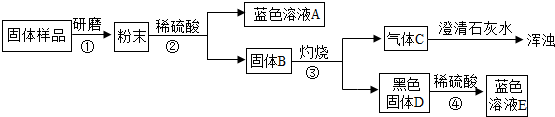
������ڰ����ܽ⡢
���� C�Ļ�ѧʽΪ
����ϣ�2���Ľ����ƶϣ�֤������
| �¶ȡ� | 0 | 20 | 40 | 60 | 80 | 100 | |
| �ܽ�ȣ�g�� | NaOH | 31 | 91 | 111 | 129 | 313 | 336 |
| Ca��OH��2 | 0.19 | 0.17 | 0.14 | 0.12 | 0.09 | 0.08 | |
�������Ƶ��ܽ�����¶ȵ����߶���������⻯�Ƶ��ܽ�����¶ȵ����߶�����
�������Ƶ��ܽ�����¶ȵ����߶���������⻯�Ƶ��ܽ�����¶ȵ����߶�����
��дһ������2����80��ʱNaOH�ı�����Һ������20�棬���Կ�����������
�������
�������
��3������20��ʱ��Ca��OH��2�ı�����Һ������Һ���������м���һ������CaO��õ�����Һ������Һ������ʱ��Һ����������������
��
��
�ң����=����������4��

������ڰ����ܽ⡢
����
����
�������������ǹ�ͬ�õ��IJ����������ձ��������������� C�Ļ�ѧʽΪ
CO2
CO2
���Ƴ�����B�к���C��Cu
C��Cu
��д��ѧʽ��������ܷ�����Ӧ�Ļ�ѧ����ʽΪCuO+H2SO4�TCuSO4+H2O
CuO+H2SO4�TCuSO4+H2O
������ϣ�2���Ľ����ƶϣ�֤������
IV
IV
��������������1�����ݱ��������������������Ƶ��ܽ�����ݽ��з������
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С���з������
��3�����������ܽ�����¶����߶���С��ͬʱ�������������ˮ���ȵ�֪ʶ���з������
��4������ĩ�м���ϡ���ᣬ��Ӧ�������Һ���壬Ҫ�����ܽ⡢���˵Ȳ�����
��������̼��ʹ����ʯ��ˮ����ǣ�����ɫ����D��ϡ���ᷴӦ���ɵ���ɫ��Һ������ͭ��Һ���ݴ˸��ݢܡ��ݵ������Ƶ�������B����ɣ�
�������ᷴӦ����Һ����ɫ����Һ��Ӧ��������ͭ����ϣ����е������Ʋ���Ʒ�ĺ������������
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С���з������
��3�����������ܽ�����¶����߶���С��ͬʱ�������������ˮ���ȵ�֪ʶ���з������
��4������ĩ�м���ϡ���ᣬ��Ӧ�������Һ���壬Ҫ�����ܽ⡢���˵Ȳ�����
��������̼��ʹ����ʯ��ˮ����ǣ�����ɫ����D��ϡ���ᷴӦ���ɵ���ɫ��Һ������ͭ��Һ���ݴ˸��ݢܡ��ݵ������Ƶ�������B����ɣ�
�������ᷴӦ����Һ����ɫ����Һ��Ӧ��������ͭ����ϣ����е������Ʋ���Ʒ�ĺ������������
����⣺��1���ɴӱ������ݿ�֪���������Ƶ��ܽ�����¶ȵ����߶���������⻯�Ƶ��ܽ�����¶ȵ����߶����٣�
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С���ʽ��»�ʹ��Һ�������ʣ�
��3������������ˮ�����ʹ��Һ�¶����ߣ������������ܽ�����¶����߶���С���������������������γɸ���ʱ�ı�����Һ������ҺҪ�ȵ���ʱ������������С���ʴ�ʱ��Һ���������������ף��ң�
��4������ĩ�м���ϡ���ᣬ��Ӧ�������Һ���壬Ҫ�����ܽ⡢���˵Ȳ�����
��������̼����ʹ����ʯ��ˮ����ǣ������Ƴ�C�Ƕ�����̼������Ӧ������ҺΪ��ɫ��Һ������ɫ��ҺΪ����ͭ��Һ������B���պ�õ��ĺ�ɫ���������ᷴӦ��õ���ɫ��Һ�������Ƴ�D������ͭ������C�Ƕ�����̼��D������ͭ����ȷ��B������ǣ�C��Cu��
�����������ͭ��ϡ���ᷴӦ����Ӧ�Ļ�ѧ����ʽΪ��CuO+H2SO4�TCuSO4+H2O��
�������������Һ����ɫ����֪����Һ�к�������ͭ����֪ԭ��ĩ��������ͭ����2���Ƶ���B����ͭ��Ҳ����ԭ��ĩ����ͭ������ԭ��ĩ����ͭ������ͭ��̼�������жϳ�����IV��ȷ��
�ʴ�Ϊ����1���������Ƶ��ܽ�����¶ȵ����߶���������⻯�Ƶ��ܽ�����¶ȵ����߶����٣�
��2�����������
��3������
��4�������ˣ�����CO2��C��Cu��CuO+H2SO4�TCuSO4+H2O������IV��
��2�������������Ƶ��ܽ�����¶Ƚ��Ͷ���С���ʽ��»�ʹ��Һ�������ʣ�
��3������������ˮ�����ʹ��Һ�¶����ߣ������������ܽ�����¶����߶���С���������������������γɸ���ʱ�ı�����Һ������ҺҪ�ȵ���ʱ������������С���ʴ�ʱ��Һ���������������ף��ң�
��4������ĩ�м���ϡ���ᣬ��Ӧ�������Һ���壬Ҫ�����ܽ⡢���˵Ȳ�����
��������̼����ʹ����ʯ��ˮ����ǣ������Ƴ�C�Ƕ�����̼������Ӧ������ҺΪ��ɫ��Һ������ɫ��ҺΪ����ͭ��Һ������B���պ�õ��ĺ�ɫ���������ᷴӦ��õ���ɫ��Һ�������Ƴ�D������ͭ������C�Ƕ�����̼��D������ͭ����ȷ��B������ǣ�C��Cu��
�����������ͭ��ϡ���ᷴӦ����Ӧ�Ļ�ѧ����ʽΪ��CuO+H2SO4�TCuSO4+H2O��
�������������Һ����ɫ����֪����Һ�к�������ͭ����֪ԭ��ĩ��������ͭ����2���Ƶ���B����ͭ��Ҳ����ԭ��ĩ����ͭ������ԭ��ĩ����ͭ������ͭ��̼�������жϳ�����IV��ȷ��
�ʴ�Ϊ����1���������Ƶ��ܽ�����¶ȵ����߶���������⻯�Ƶ��ܽ�����¶ȵ����߶����٣�
��2�����������
��3������
��4�������ˣ�����CO2��C��Cu��CuO+H2SO4�TCuSO4+H2O������IV��
�����������ѶȽϴ��漰��֪ʶ��϶ࡢ�ۺ��Խ�ǿ�����չ����ܽ�ȵ�Ӱ�����ء���Ļ�ѧ���ʡ����͵�ʵ���������������ѧ֪ʶ������ȷ�����Ĺؼ����ڣ�

��ϰ��ϵ�д�
�����Ŀ