��Ŀ����
 ��ѧ��ȤС�����о�Ӱ��������⣨H2O2���ֽ��ٶȵ����أ�����֧�Թ��зֱ����0.2�˶������� ��MnO2����ĩ��Ȼ���������IJ�ͬŨ�ȵĹ���������Һ���۲����ݲ������ٶȣ���¼���£�
��ѧ��ȤС�����о�Ӱ��������⣨H2O2���ֽ��ٶȵ����أ�����֧�Թ��зֱ����0.2�˶������� ��MnO2����ĩ��Ȼ���������IJ�ͬŨ�ȵĹ���������Һ���۲����ݲ������ٶȣ���¼���£�| ʵ����� | 1 | 2 | 3 | 4 |
| H2O2��Һ��Ũ�� | 1% | 5% | 10% | 15% |
| MnO2���� | 0.2�� | 0.2�� | 0.2�� | 0.2�� |
| ���ݲ������ٶ� | + | ++ | +++ | ++++ |
H2O2��Һ��Ũ��
H2O2��Һ��Ũ��
�Ĺ�ϵ����2��Ϊ�˼�������������Ƿ�Ϊ����������д������ķ�����
�������ǵ�ľ�������Թ��У����ľ����ȼ����֤��������Ϊ����
�������ǵ�ľ�������Թ��У����ľ����ȼ����֤��������Ϊ����
����3�����������ڴ�ʵ���е�������
��
��
���ã���Ӧǰ����������
����
����ѧ����
��ѧ����
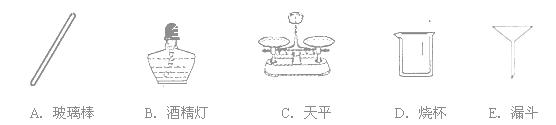
�����䣮��4��ʵ����Ϻ����ù��˷�����ʣ����Һ�еĶ������̷�ĩ���ڹ��˹����У�������������Ҫ�е���
BC
BC
������ţ���ͬ����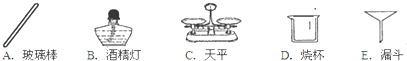
��5�����������˲��������õ�Һ���Գ��ֻ��ǣ���ԭ�������
AC
AC
��A��©���ڵ���ֽ������Ե B��©���¶˹ܿ�δ�����ձ��ڱ�
C��©����Һ�������ֽ�ı�Ե D����ֽδ����©���ڱڣ�
��������1������ʵ�����������ݿ��ǽ��
��2��������������ȼ�Խ��н��
��3�����ݶ��������ڸ�ʵ����������ý��н��
��4�����ݹ������õ����������н��
��5�����ݹ��˹����е�ע��������н��
��2��������������ȼ�Խ��н��
��3�����ݶ��������ڸ�ʵ����������ý��н��
��4�����ݹ������õ����������н��
��5�����ݹ��˹����е�ע��������н��
����⣺��1������Ŀ�е����ݿ�֪���ڶ������̵���һ��������£�˫��ˮ��Һ��������������Խ�����ݲ������ٶ�Խ�죬���Ը�ʵ���о��������ǹ�������ֽ���ٶ���˫��ˮ��Һ��Ũ�ȵĹ�ϵ��
��2����Ϊ����������ȼ�ԣ��ô����ǵ�ľ����������������Ƿ�Ϊ����������ķ������������ǵ�ľ�������Թ��У����ľ����ȼ����֤��������Ϊ������
��3�����������ڸ�ʵ���мӿ��˹������ⷴӦ�ų����������ʣ��������������ͻ�ѧ���ʲ��䣬���������ڴ�ʵ���е������Ǵ����ã�
��4���������õ������������������ձ���©��������̨�ȣ�������Ҫ��ƽ�;ƾ��ƣ�
��5�����������˲��������õ�Һ���Գ��ֻ��ǣ���ԭ�������©���ڵ���ֽ������Ե��©����Һ�������ֽ�ı�Ե����©���¶˹ܿ�δ�����ձ��ڱں���ֽδ����©���ڱڲ���������õ�Һ���Գ��ֻ��ǣ���ѡ��AC��
�ʴ�Ϊ����1��H2O2��Һ��Ũ�ȣ�
��2���������ǵ�ľ�������Թ��У����ľ����ȼ����֤��������Ϊ������
��3���� ���ã����� �� ��ѧ���� �����䣮
��4��BC��
��5��AC��
��2����Ϊ����������ȼ�ԣ��ô����ǵ�ľ����������������Ƿ�Ϊ����������ķ������������ǵ�ľ�������Թ��У����ľ����ȼ����֤��������Ϊ������
��3�����������ڸ�ʵ���мӿ��˹������ⷴӦ�ų����������ʣ��������������ͻ�ѧ���ʲ��䣬���������ڴ�ʵ���е������Ǵ����ã�
��4���������õ������������������ձ���©��������̨�ȣ�������Ҫ��ƽ�;ƾ��ƣ�
��5�����������˲��������õ�Һ���Գ��ֻ��ǣ���ԭ�������©���ڵ���ֽ������Ե��©����Һ�������ֽ�ı�Ե����©���¶˹ܿ�δ�����ձ��ڱں���ֽδ����©���ڱڲ���������õ�Һ���Գ��ֻ��ǣ���ѡ��AC��
�ʴ�Ϊ����1��H2O2��Һ��Ũ�ȣ�
��2���������ǵ�ľ�������Թ��У����ľ����ȼ����֤��������Ϊ������
��3���� ���ã����� �� ��ѧ���� �����䣮
��4��BC��
��5��AC��
������ͨ���ش���֪����Ӱ��������ⷴӦ�ٶȵ����ء���������ȼ���Լ������е�ע����������Ա�ʵ�飬�ڷ���ʵ�����ݵĻ����ϵõ���ȷ���ۣ��Ƕ�ѧ�����������Ŀ��飮

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д�
�����Ŀ
��ѧ��ȤС�����о�Ӱ�����
����(H2O2)�ֽ��ٶȵ����ء�����֧�Թ��зֱ����0.2�˶������� (MnO2)��ĩ��Ȼ���������IJ�ͬ�������������Ĺ���������Һ���۲����ݲ������ٶȣ���¼���£�
 |
(1)��ʵ���о��������ǣ���������ֽ���ٶ���_________�Ĺ�ϵ�� ��
(2)Ϊ�˼�������������Ƿ�Ϊ����������д������ķ�����____________________��
(3)ʵ����ϣ������ù��˷�����ʣ����Һ�еĶ������̷�ĩ���ڹ��˹����У�![]() ������������Ҫ����______________��
������������Ҫ����______________��
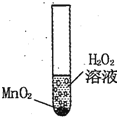 ��ѧ��ȤС�����о�Ӱ��������⣨H2O2���ֽ��ٶȵ����أ�����֧�Թ��зֱ����0.2g��������
��ѧ��ȤС�����о�Ӱ��������⣨H2O2���ֽ��ٶȵ����أ�����֧�Թ��зֱ����0.2g��������