��Ŀ����
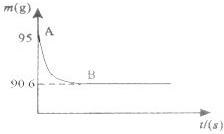 ��ѧ����ʦָ��ij��ѧ��ȤѧϰС�������һ����Ȥ��ʵ��̽�����ⶨ�����ǵ���Ҫ�ɷ�̼��Ƶ�������������ȡ15g�����ǣ����飬�����ձ��У�Ȼ�������м���80gϡ���ᣬʹ֮ǡ����ȫ��Ӧ�������������ʲ���ϡ���ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������ˮ�����Ļӷ������Լ��㣺
��ѧ����ʦָ��ij��ѧ��ȤѧϰС�������һ����Ȥ��ʵ��̽�����ⶨ�����ǵ���Ҫ�ɷ�̼��Ƶ�������������ȡ15g�����ǣ����飬�����ձ��У�Ȼ�������м���80gϡ���ᣬʹ֮ǡ����ȫ��Ӧ�������������ʲ���ϡ���ᷴӦ��������ձ��еķ�Ӧʣ�����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ������ˮ�����Ļӷ������Լ��㣺��1�������������������Ϊ
��2������ü�������̼��Ƶ�������������Ҫ��д��������̣�
��������1�����ݷ�Ӧǰ�����������Լ������ɶ�����̼��������
��2��̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���������ɶ�����̼�����������Լ���̼��Ƶ��������Ӷ����Լ��㼦������̼��Ƶ�����������
��2��̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼���������ɶ�����̼�����������Լ���̼��Ƶ��������Ӷ����Լ��㼦������̼��Ƶ�����������
����⣺��1�����ɵĶ�����̼����Ϊ��15g+80g-90.6g=4.4g��
���4.4��
��2����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g
=
x=10g
̼��Ƶ���������Ϊ��
��100%��66.7%��
�𣺸ü�������̼��Ƶ���������Ϊ66.7%��
���4.4��
��2����̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 4.4g
| 100 |
| x |
| 44 |
| 4.4g |
x=10g
̼��Ƶ���������Ϊ��
| 10g |
| 15g |
�𣺸ü�������̼��Ƶ���������Ϊ66.7%��
���������������������뻯ѧ����ʽ���ۺϼ��㣬����Ҫ��ȷд������ʽ���ٸ���������ϸ����������ϵ��������㣬������⣮

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ