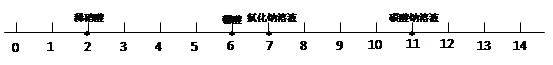��Ŀ����
��2010���뽭����5�֣������ܽ���ѧϰ��ѧ����Ҫ������С��ͬѧ��ͼl�ܽ���NaOH��������ѧ���ʣ���NaOH�����������ܹ�������ѧ��Ӧ����
��l��Ϊ����֤��Ӧ�٣�С������ɫ������Һ����NaOHҺ�У���Һ��� ɫ��
��2�����ݷ�Ӧ��˵��NaOH�����ܷⱣ�棬�����ڿ�����Ҫ���ʣ��仯ѧ��Ӧ����ʽΪ��
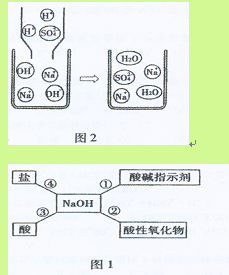
��3��С���ûչ�ʾ��ͼ��ͼ2����˵����Ӧ�۵ķ������û�ѧ��
Ӧ����ʽ��ʾΪ��
�Ӹ�ͼ���Կ�������Ӧǰ��û�з����仯�Ļչ�������
��4��Ϊ����֤��Ӧ���ܹ���������ѡ��������� ��

��l��Ϊ����֤��Ӧ�٣�С������ɫ������Һ����NaOHҺ�У���Һ��� ɫ��
��2�����ݷ�Ӧ��˵��NaOH�����ܷⱣ�棬�����ڿ�����Ҫ���ʣ��仯ѧ��Ӧ����ʽΪ��
��3��С���ûչ�ʾ��ͼ��ͼ2����˵����Ӧ�۵ķ������û�ѧ��
Ӧ����ʽ��ʾΪ��
�Ӹ�ͼ���Կ�������Ӧǰ��û�з����仯�Ļչ�������
��4��Ϊ����֤��Ӧ���ܹ���������ѡ��������� ��
| A��Na2CO3 | B��HCl | C��CuSO4 | D��NaCl |
��l�� �� ��2�� CO2��2NaOH=Na2CO3��H2O
��3��2NaOH��H2SO4=Na2SO4��2H2O Na����SO42�� ��4�� C
��3��2NaOH��H2SO4=Na2SO4��2H2O Na����SO42�� ��4�� C
�������Ը���NaOH�����ָʾ��������ɫ��Ӧ��������εķ�Ӧ�йع��ɽ��з��������ݸ��ֽⷴӦ����������ѧ����ʽ����дҪ����з������
��𣺽⣺��1����̪�����ɫ�������ɫ��
��2���������ƿ��ԺͿ����еĶ�����̼��Ӧ�����ʣ�CO2+2NaOH=Na2CO3+H2O��
��3��ͨ��ͼʾ�ɿ�����Ӧ��������ˮ���������������û�в��뷴Ӧ��H2SO4+2NaOH=Na2SO4+2H2O��
��4����������Ҫ��֤���εķ�Ӧ������B���ԣ����ݸ��ֽⷴӦ�������������ɳ����������ˮ������ѡ��C
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
�ʴ�Ϊ����1����
��2��CO2+2NaOH=Na2CO3+H2O
��3��H2SO4+2NaOH=Na2SO4+2H2O��SO42-��Na+
��4��C
��𣺽⣺��1����̪�����ɫ�������ɫ��
��2���������ƿ��ԺͿ����еĶ�����̼��Ӧ�����ʣ�CO2+2NaOH=Na2CO3+H2O��
��3��ͨ��ͼʾ�ɿ�����Ӧ��������ˮ���������������û�в��뷴Ӧ��H2SO4+2NaOH=Na2SO4+2H2O��
��4����������Ҫ��֤���εķ�Ӧ������B���ԣ����ݸ��ֽⷴӦ�������������ɳ����������ˮ������ѡ��C
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
�ʴ�Ϊ����1����
��2��CO2+2NaOH=Na2CO3+H2O
��3��H2SO4+2NaOH=Na2SO4+2H2O��SO42-��Na+
��4��C

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ

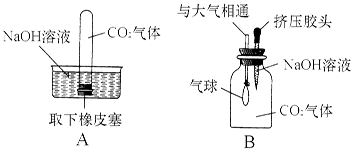
 ��
��