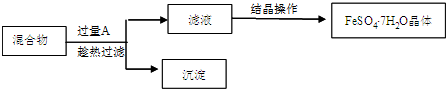
��Ŀ����
ij��ѧ����С���ú�ͭ90%�ķ�ͭм��ȡ��������ˮ������ͭ������ͭм����ϡ�����У����Ȳ����Ϲ�������������������±���������ͭ��
��1��д����������ͭ�Ļ�ѧ����ʽ
��2��˵��������һ����;
��1��д����������ͭ�Ļ�ѧ����ʽ
2Cu+O2
2CuO��CuO+H2SO4�TCuSO4+H2O
| ||
2Cu+O2
2CuO��CuO+H2SO4�TCuSO4+H2O
��
| ||
��2��˵��������һ����;
���Ʋ�����Һ������ɱ������
���Ʋ�����Һ������ɱ������
����������1������ͭ������ͭ�Ļ�ѧ���ʽ��ͭ����������Ӧ��������ͭ������ͭ�������ᷴӦ��������ͭ��
��2���������������Ʋ�����Һ��
��2���������������Ʋ�����Һ��
����⣺��1��ͭ����������������������ͭ������ͭ�����ᷴӦ��������ͭ����ѧ����ʽΪ��2Cu+O2
2CuO��CuO+H2SO4�TCuSO4+H2O
��2���������Ժ���ʯ��һ�������Ʋ�����Һ������ɱ��������
�ʴ�Ϊ��
��1��2Cu+O2
2CuO��CuO+H2SO4�TCuSO4+H2O
��2�����Ʋ�����Һ������ɱ������
| ||
��2���������Ժ���ʯ��һ�������Ʋ�����Һ������ɱ��������
�ʴ�Ϊ��
��1��2Cu+O2
| ||
��2�����Ʋ�����Һ������ɱ������
��������ȷ�������ʡ�����������Ļ�ѧ�����ǽ����ؽ�����������������Ӧ���ɽ�����������������������ᷴӦ��

��ϰ��ϵ�д�
�����Ŀ