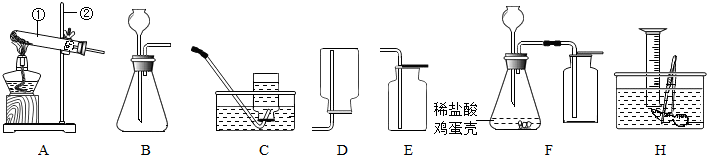
��Ŀ����
�ձ���װ��133.8��ϡ���������ͭ�Ļ����Һ���������Һ�м���10%������������Һ���õ��������������£�
��1��д�����з�����Ӧ�Ļ�ѧ����ʽ ��
��2���û����Һ������ͭ������Ϊ �ˣ�
��3������������������Һ ��ʱ���õ��������������ٱ仯��
��4���г����ԭ�����Һ�к�������������x���ı���ʽ ��
��5���������Һ�м���10%������������Һֱ������ǡ����ȫʱ������Һ�������Ƶ��������� ��
| ��������������Һ��������g�� | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
| ���ɳ�������������g�� | 0.0 | 2.6 | 8.6 | 9.8 | 9.8 |
��2���û����Һ������ͭ������Ϊ
��3������������������Һ
��4���г����ԭ�����Һ�к�������������x���ı���ʽ
��5���������Һ�м���10%������������Һֱ������ǡ����ȫʱ������Һ�������Ƶ���������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
�����������������ͭ�Ļ����Һ�μ�����������Һ�����ᡢ����ͭ�������������Ʒ�����Ӧ����������Ĵ�����������������ͭ��������������ͭ�����������ᷴӦ����ܲ���������ͭ���������Լ�¼�����У�����50.0g����������Һʱ��������������Ϊ0�����ڼ�������������Һ200.0g�Ժ�����������ٱ仯��˵������ͭҲ����ȫ��Ӧ�������ɳ��������ֵΪ9.8g��
��������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ���ɳ���������ͭ�������ɼ�������Һ������ͭ��������
��������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ���ɳ���������ͭ�������ɼ�������Һ������ͭ��������
����⣺��1�����з�����Ӧ�Ļ�ѧ����ʽ��H2SO4+2NaOH=Na2SO4+2H2O��CuSO4+2NaOH=Na2SO4+Cu��OH��2����
��2���ɼ�¼���ݱ���֪��������ͭ��ȫ��Ӧ��������ɫ����9.8g��
����Һ������ͭ������Ϊx
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
x 9.8g
=
x=16g
�𣺸û����Һ������ͭ��������16�ˣ�
��3���������������ٱ仯ʱ����������������Һ����Ϊ��150g+
��(9.8g-8.6g)=160g
��4���������ĵ�����������Һ����Ϊ��100g-
��2.6g=78.3g
ԭ�����Һ�к�����������x
H2SO4+2NaOH=Na2SO4+2H2O
98 80
x 78.3g��10%
ԭ�����Һ�к�������������x���ı���ʽ��
=
��5���裬����10%������������Һֱ������ǡ����ȫʱ������Һ�������Ƶ�����Ϊy��
2NaOH��Na2SO4
80 142
160g��10% y
=
y=28.4g
����ǡ����ȫʱ������Һ�������Ƶ�����������
��100%=10%��
�𰸣���1��H2SO4+2NaOH=Na2SO4+2H2O��CuSO4+2NaOH=Na2SO4+Cu��OH��2����
��2��16��
��3��160��
��4��
=
��
��5��10%��
��2���ɼ�¼���ݱ���֪��������ͭ��ȫ��Ӧ��������ɫ����9.8g��
����Һ������ͭ������Ϊx
CuSO4+2NaOH=Na2SO4+Cu��OH��2��
160 98
x 9.8g
| 160 |
| x |
| 98 |
| 9.8g |
x=16g
�𣺸û����Һ������ͭ��������16�ˣ�
��3���������������ٱ仯ʱ����������������Һ����Ϊ��150g+
| 150g-100g |
| 8.6g-2.6g |
��4���������ĵ�����������Һ����Ϊ��100g-
| (150g-100g) |
| 8.6g-2.6g |
ԭ�����Һ�к�����������x
H2SO4+2NaOH=Na2SO4+2H2O
98 80
x 78.3g��10%
ԭ�����Һ�к�������������x���ı���ʽ��
| 98 |
| x |
| 80 |
| 78.3%��10% |
��5���裬����10%������������Һֱ������ǡ����ȫʱ������Һ�������Ƶ�����Ϊy��
2NaOH��Na2SO4
80 142
160g��10% y
| 80 |
| 160g��10% |
| 142 |
| y |
y=28.4g
����ǡ����ȫʱ������Һ�������Ƶ�����������
| 28.4g |
| 133.8g+160g-9.8g |
�𰸣���1��H2SO4+2NaOH=Na2SO4+2H2O��CuSO4+2NaOH=Na2SO4+Cu��OH��2����
��2��16��
��3��160��
��4��
| 98 |
| x |
| 80 |
| 78.3%��10% |
��5��10%��
������ѧ��Ӧ��Ϥ���û�ѧ����ʽ�����˼·��ʽ��������ǡ�÷�Ӧ��Ԫ���غ�ͷ������������

��ϰ��ϵ�д�
 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
�����е�����������Ⱦ���ǣ�������
| A��SO2��CO��NO2 |
| B��SO2��CO2��NO2 |
| C��SO2��NO2��O2 |
| D��SO2��N2��NO2 |
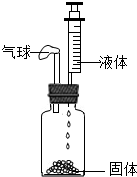 ����ͼ��ʾ���ܱ�ʵ��װ�ã����ܹ�ʹ���������������ǣ�������
����ͼ��ʾ���ܱ�ʵ��װ�ã����ܹ�ʹ���������������ǣ�������| A�����壺�������̣�Һ�壺˫��ˮ��Һ |
| B�����壺��ʯ�ң� Һ�壺ˮ |
| C�����壺̼��ƣ� Һ�壺ϡ���� |
| D�����壺�Ȼ��ƣ� Һ�壺ˮ |
 ��γͬѧ�����ǵĺ�ˮ��Ʒ�����ձ��У��ȼ���������ĩ�����ܽ⣬����һ�������ͼ��ʾ��װ�ý��й��ˣ����ʣ�
��γͬѧ�����ǵĺ�ˮ��Ʒ�����ձ��У��ȼ���������ĩ�����ܽ⣬����һ�������ͼ��ʾ��װ�ý��й��ˣ����ʣ� ��ȤС���ͬѧ��ʵ������ȡ������̼������������ƿ��ʣ������Է�Һ���Ľ���ʵ��װ�ã���ͼ��ʾ���������飺�����ڼ���ƿ�г���������̼��Ȼ��Һ©���ڵ�Ũ����������Һ����ƿ�ڣ������Թ۲쵽��������
��ȤС���ͬѧ��ʵ������ȡ������̼������������ƿ��ʣ������Է�Һ���Ľ���ʵ��װ�ã���ͼ��ʾ���������飺�����ڼ���ƿ�г���������̼��Ȼ��Һ©���ڵ�Ũ����������Һ����ƿ�ڣ������Թ۲쵽�������� ijУʵ���ҵķ�Һ�����ռ���ѧ����ʵ������ȡ������̼������ķ�Һ��ͬѧ����̽����Һ�����ʵijɷ֣�����һͬ����̽�����ش��������⣺
ijУʵ���ҵķ�Һ�����ռ���ѧ����ʵ������ȡ������̼������ķ�Һ��ͬѧ����̽����Һ�����ʵijɷ֣�����һͬ����̽�����ش��������⣺