��Ŀ����
����Ŀ��(7��)ʵ�������и�����ء�ϡ���ᡢϡ���ᡢʯ��ʯ�������������������

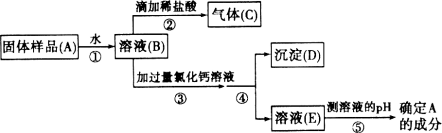
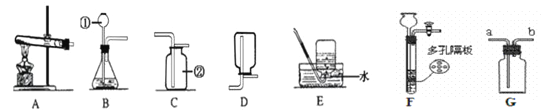
��1����������ҩƷ��ȡ���ռ�������̼���壬Ӧѡ����ͼ�����еĢ�_________(����ĸ)����Ӧ�Ļ�ѧ����ʽΪ��________________��
��2�������䲿��������������ҩƷ������ȡ�������뽫�Ҳ���ȡ���ռ�������װ��ͼ����������
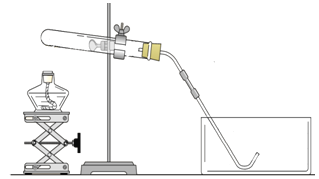
��3��fͼ���������װ�õ������Ե�ʾ��ͼ��������ס�Թ���ڣ����װ�õ�������������ͼ�п��Թ۲쵽�������Ǣ�_______________����ԭ����������ס�Թܣ��Թ��������¶Ȣ�____________������ѹǿ��_______________����ѹǿ��������¹۲쵽���������ɿ�����ɹ۲쵽��_____________��
���𰸡���1��bc�� CaCO3 + 2HCl==CaCl2 + CO2�� + H2O ��2���Թܿ�û��������û��װ��ˮ�ļ���ƿ��ˮ����û��ˮ ��3���ձ��л�ð�����ݣ����ߣ�����ձ��е�����Һ���������γ�ˮ����
��������
�����������������ҩƷ��ȡ���ռ�������̼���壬Ӧѡ����ͼ�����е�bc����Ϊ����Ҫ�������Է���װ��Ϊb����Ӧ�Ļ�ѧ����ʽΪCaCO3 + 2HCl==CaCl2 + CO2�� + H2O��ͼ�л�û�����ƣ������Թܿ�û��������û��װ��ˮ�ļ���ƿ��ˮ����û��ˮ��fͼ���������װ�õ������Ե�ʾ��ͼ��������ס�Թ���ڣ����װ�õ�������������ͼ�п��Թ۲쵽���������ձ��л�ð�����ݣ���ԭ����������ס�Թܣ��Թ��������¶����ߣ�����ѹǿ�����ѹǿ��������¹۲쵽���������ɿ�����ɹ۲쵽�ձ��е�����Һ���������γ�ˮ����