��Ŀ����
��ͼ��ʾ��ijͬѧ��һ����̼����������ԭ����ͭ��ȡ����ͭ�������������̽�����̣����ش��������⡣
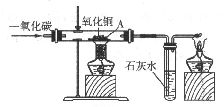
��1����д��һ����̼������ͭ��Ӧ���ɽ���ͭ�Ͷ�����̼�Ļ�ѧ����ʽ��_____________��
��2����װ���У��ұߵľƾ��Ƶ�������_______________________________________��
��3��ʵ�������ͬѧ��A�������������ɽ���̽�����������ϵ�֪�����ڸ�����ͭ�����¶Ȳ�����ͨ��һ����̼���㣬��Ӧ����������ͭ�������������м����������ͭ����ѧʽΪCu2O����������ͭ��һ�ֺ�ɫ���壬������ˮ������ϡ���ᷢ�����·�Ӧ��
Cu2O+H2SO4==CuSO4+Cu+H2O
����������ͭ��ͭԪ�صĻ��ϼ�Ϊ_____________��
�ڲ��������������ļ��ֿ���?���û�ѧʽ��ʾ��__________________________________��
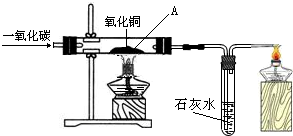
���������ʵ�飬֤������ͭ�Ƿ�ȫ��ת��Ϊ����ͭ��
ʵ�����ݺͲ��� | �۲쵽��ʵ������ͽ��� |
��1��CO+CuO![]() Cu+CO2
Cu+CO2
��2����δ�μӷ�Ӧ��COȼ�յ�����ֹ��Ⱦ����
��3����+1�� ��a��CuO��Cu2O������ͭ��������ͭ����b��Cu2O��������ͭ����c��Cu��CuO��ͭ������ͭ����d��Cu��Cu2O ��ͭ��������ͭ����e��Cu��Cu2O��CuO��ͭ��������ͭ������ͭ����f��Cu��ͭ��
��
| ȡ���������������Թ��У���������ϡ���ᣬ����� ����ͼװ�ã���ͨ��CO��ͬʱ����������A���IJ������塣�� | ����Һ������ɫ��֤������ͭȫ��ת��Ϊ����ͭ��������Һ�����ɫ��֤������ͭû��ȫ��ת��Ϊ����ͭ���� ����ʯ��ˮ������ǣ�֤������ͭȫ��ת��Ϊ����ͭ����ʯ��ˮ����ǣ�֤������ͭû��ȫ��ת��Ϊ����ͭ���� |

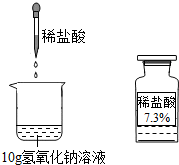 ��ͼ��ʾΪijͬѧ��ϡ����ⶨһδ֪Ũ�ȵ�����������Һ��ʵ�飬��ǡ����ȫ��Ӧʱ������ϡ���������Ϊ5g������������Һ�к����������Ƶ������Ƕ��٣�
��ͼ��ʾΪijͬѧ��ϡ����ⶨһδ֪Ũ�ȵ�����������Һ��ʵ�飬��ǡ����ȫ��Ӧʱ������ϡ���������Ϊ5g������������Һ�к����������Ƶ������Ƕ��٣� ��ͼ��ʾΪijͬѧ��ϡ����ⶨһδ֪Ũ�ȵ�����������Һ��ʵ�飬��ǡ����ȫ��Ӧʱ������ϡ���������Ϊ5g������������Һ�к����������Ƶ������Ƕ��٣�
��ͼ��ʾΪijͬѧ��ϡ����ⶨһδ֪Ũ�ȵ�����������Һ��ʵ�飬��ǡ����ȫ��Ӧʱ������ϡ���������Ϊ5g������������Һ�к����������Ƶ������Ƕ��٣�