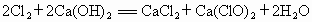��Ŀ����
�����ֺ����������dz������ֻ�ѧ���ʴ�����ˮ��һ������������������ˮ����������ʹ���������һ����Ư�ۣ�����ɱ��������Ư�۵���Ҫ�ɷ���Ca��ClO��2�������ɻ���������������ʯ����ԭ���Ƶõģ�����������Ҫ�ɷ�ռ65%��Ư��110g����ѧ����ʽΪ2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O������㣺
��1��Ư������Ҫ�ɷֵ�����Ϊ���٣�
��2������ˮ�������ƹ�ʹ��ClO2����Ư�ۣ��Ʋ�ClO2����ɱ���������õ�Ԫ���ǣ������ƣ�______��
��3�����Ƶ�������Ҫ�ɷ�ռ65%��Ư��110g��������������Ϊ���٣�
���𰸡���������1������Ư�۵���Ҫ�ɷֵ����������Լ�Ư�۵�����������ɣ�
��2������ClO2�ܴ���Ư�ۣ������������ߺ��е�Ԫ�����������ɣ�
��3�����÷�Ӧ�Ļ�ѧ����ʽ��������Ҫ�ɷֵ������ɼ�������������������
����⣺��1��Ư������Ҫ�ɷֵ�����=110g×65%=71.5g��
��2�����ݸ���ClO2�ܴ���Ư�ۣ���Ư�۵���Ҫ�ɷ���Ca��ClO��2�����Կ�������ɱ���������õ�Ԫ�������߾����е�Ԫ�أ�������Ԫ�أ�
��2����������������Ϊx
2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O
142 143
x 71.5g
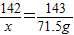
x=71g
�𣺣�1��Ư������Ҫ�ɷֵ�����Ϊ71.5g��
��2����Ԫ�أ�
��3�����Ƶ�������Ҫ�ɷ�ռ65%��Ư��110g��������������Ϊ71g��
������������Ҫ�Ǹ��ݻ�ѧ����ʽ�ļ����⣬���Ҵ�������ķ���ʽ�Ѿ����������Խ������Ѷȣ����ݼ���Ҳ�����ӣ���һ���Ƚϼļ����⣮
��2������ClO2�ܴ���Ư�ۣ������������ߺ��е�Ԫ�����������ɣ�
��3�����÷�Ӧ�Ļ�ѧ����ʽ��������Ҫ�ɷֵ������ɼ�������������������
����⣺��1��Ư������Ҫ�ɷֵ�����=110g×65%=71.5g��
��2�����ݸ���ClO2�ܴ���Ư�ۣ���Ư�۵���Ҫ�ɷ���Ca��ClO��2�����Կ�������ɱ���������õ�Ԫ�������߾����е�Ԫ�أ�������Ԫ�أ�
��2����������������Ϊx
2Cl2+2Ca��OH��2=CaCl2+Ca��ClO��2+2H2O
142 143
x 71.5g
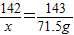
x=71g
�𣺣�1��Ư������Ҫ�ɷֵ�����Ϊ71.5g��
��2����Ԫ�أ�
��3�����Ƶ�������Ҫ�ɷ�ռ65%��Ư��110g��������������Ϊ71g��
������������Ҫ�Ǹ��ݻ�ѧ����ʽ�ļ����⣬���Ҵ�������ķ���ʽ�Ѿ����������Խ������Ѷȣ����ݼ���Ҳ�����ӣ���һ���Ƚϼļ����⣮

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ
 ��
��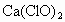 �������ɻ���������������ʯ����ԭ���Ƶõģ���ѧ����ʽ
�������ɻ���������������ʯ����ԭ���Ƶõģ���ѧ����ʽ