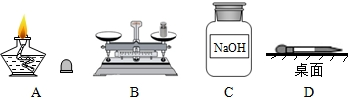
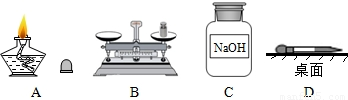
��Ŀ����
��1�����ջ�ѧʵ���г�����������������;�������ڿ�չ��ѧѧϰ���о���
����a���ձ���b��©����c����ͷ�ιܡ� d����Ͳ�� e����ƽ��������ĸ��գ�
������ƽ�����׳��⡢�и�ʴ�Ե�ҩƷ�����______�
����ȡ��μ�����Һ�������______��
��2���淶��ʵ�������ʵ��ɹ���ǰ�ᣬ��ش�
����ȡ8mLϡ���ᣬӦѡ��______mL����Ͳ��
�ڽ�ͷ�ι��ù���Ӧ______����ȥ��ȡ����ҩƷ��
�۲����ܲ��������Ƥ�����ȰѲ����ܵ�һ��______��Ȼ����������ת�����룮
��3���ƾ�����ʵ�����г��õļ���������ijС��ͬѧ�Ծƾ��ƻ����¶Ƚ�������̽����
ȡһ�������סһ��Ѹ��ƽ����ƾ��ƻ����У�l��2s��ȡ�����۲쵽λ������IJ�������̼��������������ó����ۣ�______�¶���ߣ�д��̼�ڿ�����ȼ�յ����ֱ���ʽ��______��
��4��ijͬѧ���Թܸ����ʼ���ʱ��ʵ���ȴ�����Թ������ѣ������������һ�£�����Թ����ѵ�ԭ�������______��
______������д���㣩��
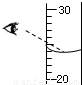
��5����ͼ��ʾ����ijͬѧ��50mL��Ͳ��ȡһ�����Һ��IJ�����������ϸ�۲��ͼ���ж���Ͳ��Һ������ʵ����______ ������ڡ����ڻ�С�ڣ�25mL��
��6������ѧʵ��Ҫ�������õ�����ϰ�ߣ���ijͬѧ��ʵ���������������ͼ���ã�������ȷ��һ����______��������ţ�

�⣺��1��������ƽ�����׳��⡢�и�ʴ�Ե�ҩƷ������ձ������������ȡ��μ�����Һ��������ǽ�ͷ�ιܣ�
��2������ȡ8mLϡ���ᣬ������Ͳ��ѡ�÷���Ӧѡ�� 10mL����Ͳ���ڽ�ͷ�ι��ù���Ӧ������ˮ��ϴ����ȥ��ȡ����ҩƷ���۲����ܲ��������Ƥ�����ȰѲ����ܵ�һ�� ��ˮʪ��Ȼ����������ת�����룻
��3������ʵ�����������IJ�������̼�����ó����ۣ����� �¶���ߣ�̼�ڿ�����ȼ�յ����ֱ���ʽ��̼+���� ������̼��
������̼��
��4�����Թܸ����ʼ���ʱ���Թ����ѵ�ԭ��϶࣬���磺�������ˮ���ܵӴ���о�����Թ�������ϴ��δԤ�Ⱦͼ��ȵ�
��5����ͼ�ǿ�֪�������������ڸ��Ӷ�������Ͳ��Һ������ʵ����С��25mL��
��6��A���ƾ��������Ӧ�����õ�ñ��������A����
B����������ƽ����ҩƷ��Ӧ������Żص�������ڣ����ԣ�B����
C��ȡ���Լ���Ӧ�����������ӣ����ԣ�C��ȷ��
D����ͷ�ι�������ܷŵ������ϣ����ԣ�D����
�ʴ�Ϊ����1����a ��c ��2����10 ��������ˮ��ϴ ����ˮʪ��3������ ̼+����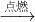 ������̼��
������̼��
��4���������ˮ���ܵӴ���о������5��С�ڣ���6��C��
��������1�����ݳ���������ʹ��ע�����������
��2������ʵ��IJ�������������;������
��3������ʵ�����������ó����ۣ����ݷ�Ӧд������ʽ��
��4�����ݸ��Թܵ����ʼ���ʱ��ע����������Թ����ѵ�ԭ��
��5��������Ͳ��ʹ��ע�����������
��5������ʵ��IJ���Ҫ�۲�ͼʾ��������������
��������dz�����������;��ע����������ɱ���Ĺؼ���
��2������ȡ8mLϡ���ᣬ������Ͳ��ѡ�÷���Ӧѡ�� 10mL����Ͳ���ڽ�ͷ�ι��ù���Ӧ������ˮ��ϴ����ȥ��ȡ����ҩƷ���۲����ܲ��������Ƥ�����ȰѲ����ܵ�һ�� ��ˮʪ��Ȼ����������ת�����룻
��3������ʵ�����������IJ�������̼�����ó����ۣ����� �¶���ߣ�̼�ڿ�����ȼ�յ����ֱ���ʽ��̼+����
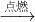 ������̼��
������̼����4�����Թܸ����ʼ���ʱ���Թ����ѵ�ԭ��϶࣬���磺�������ˮ���ܵӴ���о�����Թ�������ϴ��δԤ�Ⱦͼ��ȵ�
��5����ͼ�ǿ�֪�������������ڸ��Ӷ�������Ͳ��Һ������ʵ����С��25mL��
��6��A���ƾ��������Ӧ�����õ�ñ��������A����
B����������ƽ����ҩƷ��Ӧ������Żص�������ڣ����ԣ�B����
C��ȡ���Լ���Ӧ�����������ӣ����ԣ�C��ȷ��
D����ͷ�ι�������ܷŵ������ϣ����ԣ�D����
�ʴ�Ϊ����1����a ��c ��2����10 ��������ˮ��ϴ ����ˮʪ��3������ ̼+����
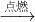 ������̼��
������̼����4���������ˮ���ܵӴ���о������5��С�ڣ���6��C��
��������1�����ݳ���������ʹ��ע�����������
��2������ʵ��IJ�������������;������
��3������ʵ�����������ó����ۣ����ݷ�Ӧд������ʽ��
��4�����ݸ��Թܵ����ʼ���ʱ��ע����������Թ����ѵ�ԭ��
��5��������Ͳ��ʹ��ע�����������
��5������ʵ��IJ���Ҫ�۲�ͼʾ��������������
��������dz�����������;��ע����������ɱ���Ĺؼ���

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ