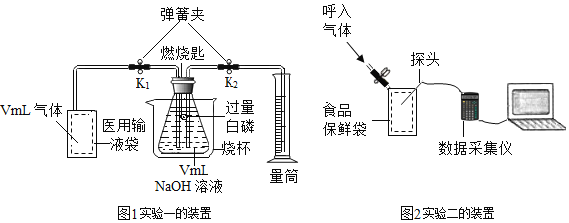
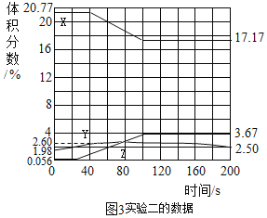
��Ŀ����
����Ŀ����Һ���ճ������ũҵ�����Ϳ�ѧ�о��о��й㷺����;��
��1��������ˮ��ҽ���ϳ��õ�һ����Һ����������_____���ѧʽ����
��2����ʢ��ˮ���ձ��м�������ij�����ʣ��γ���Һ�Ĺ������¶Ƚ��ͣ�����������___������ĸ����
A���Ȼ��� B������� C����������
��3��ijͬѧ��ʵ�������Ȼ��ƹ��������ˮ����50g��������Ϊ6%���Ȼ�����Һʱ����Ҫ�Ȼ��Ƶ�����Ϊ__________g���漰����ʵ�鲽�裺���ܽ� �ڳ�������ȡ�ۼ����װ���Լ�ƿ���ñ�ǩ������������Һ��ȷ��ʵ�鲽��˳����___________________������ţ���
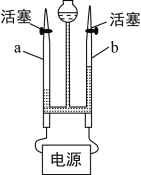
��4��ijע����ҩҺ�����Ʒ������£�
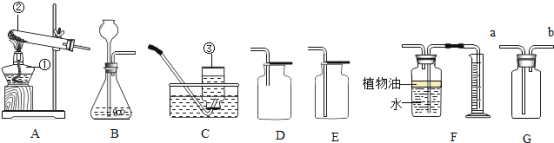
�ٰ�1.0gҩƷ����ˮ���Ƴ�4.0mL��Һa��
��ȡ0.1mL��Һa����ˮϡ����1.0mL������Һb��
��ȡ0.1mL��Һb����ˮϡ����1.0mL������Һc��
��ȡ0.4mL��Һc����ˮϡ����1.0mL������Һd��
�������������ƹ�����ҩҺ��ϡ�����ܶȶ��ɽ��ƿ���1g/cm3�������յõ���ҩҺ����Һd�������ʵ���������Ϊ________��
���𰸡�NaClB3���ۢڢ٢�0.1%
��������
��1��������ˮ��ҽ���ϳ��õ�һ����Һ�����������Ȼ��ƣ���ѧʽΪNaCl��
��2��A���Ȼ�������ˮ���¶ȼ���û�б仯��ѡ�����B�����������ˮ�����������¶Ƚ��ͣ�ѡ����ȷ��C��������������ˮ�ų��������¶����ߣ�ѡ����ʱ���ѡB��
��3���Ȼ��Ƶ�����=50g��6%=3g��ʵ����������Һ��һ�㲽��Ϊ���㡢��������ȡ���ܽ⡢װ���Լ�ƿ���ñ�ǩ����˳��Ϊ�ۢڢ٢ܣ�
��4���������֪��ҩҺ��ϡ�����ܶȶ��ɽ��ƿ���1g/cm3�����У�
1.0gҩƷ����ˮ���Ƴ�4.0mL��Һ����a��Һ��������������=![]() ��100%=25%����Һb��������������=
��100%=25%����Һb��������������=![]() ��100%=2.5%����Һc��������������=
��100%=2.5%����Һc��������������=![]() =0.25%����Һd��������������=
=0.25%����Һd��������������=![]() ��100%=0.1%��
��100%=0.1%��

 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д� ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�