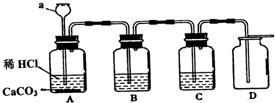
��Ŀ����
 �ش��������⣺
�ش��������⣺��1������������
ԭ��
ԭ��
���ԭ�ӡ��������ӡ������ӡ�����ͬ�����ɵģ��Ȼ�����������
����
���ɵ���2������2����λ��������ӵĽṹʾ��ͼ
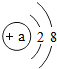 ��a=
��a=12
12
�������ΪMg2+
Mg2+
���γɸ����ӵ�ԭ�ӹ���3
3
�����Ӳ㣮��3���Ȼ�����һ����ɫ�д̼�����ζ�����壬�߽�ʵ���Ҿ��ŵ���������ζ���ӷ��ӵĽǶȽ�����������
�Ȼ�����������ڲ��ϵ��˶���
�Ȼ�����������ڲ��ϵ��˶���
�����������ӡ�ԭ�ӡ����Ӷ��ǹ������ʵ����������������ص㣺����С�����ڲ��ϵ��˶��ţ�֮�䶼�м������ԭ�ӹ��ɵ������������ࣺ�ٽ������ʣ��ڷǽ�����̬���ʣ���ϡ�����壬�������ӻ�����������������������γɣ���������ӹ��ɵ�������һ�����н���Ԫ��Ҳ�зǽ���Ԫ�أ�����������������������������������������������͵�����֮���������������������������������������ǵ�������������֮�
����⣺��1�����ǽ������ʣ�����ԭ��ֱ�ӹ��ɵģ��Ȼ������������Ӻ������ӹ��ɵģ��ʴ�Ϊ��ԭ�ӣ�����
��2������2����λ�����������ʧȥ2�����ӣ����ڵ�����Ϊ10����������Ϊ10+2=12��˵����Ϊ���������ӣ������Ǵ�������λ����ɵ�þ���ӣ�þ���ӵ�ԭ�ӹ���3�����Ӳ㣮�ʴ�Ϊ��12��Mg2+��3��
��3���Ȼ�����һ����ɫ�д̼�����ζ�����壬�߽�ʵ���Ҿ��ŵ���������ζ��˵���Ȼ�������ڲ��ϵ��˶��ţ��ʴ�Ϊ���Ȼ�����������ڲ��ϵ��˶��ţ�
��2������2����λ�����������ʧȥ2�����ӣ����ڵ�����Ϊ10����������Ϊ10+2=12��˵����Ϊ���������ӣ������Ǵ�������λ����ɵ�þ���ӣ�þ���ӵ�ԭ�ӹ���3�����Ӳ㣮�ʴ�Ϊ��12��Mg2+��3��
��3���Ȼ�����һ����ɫ�д̼�����ζ�����壬�߽�ʵ���Ҿ��ŵ���������ζ��˵���Ȼ�������ڲ��ϵ��˶��ţ��ʴ�Ϊ���Ȼ�����������ڲ��ϵ��˶��ţ�
�����������ѶȲ��Ǻܴ���Ҫ�����˶������ʵ��������ӡ�ԭ�ӡ����ӣ�����ѧ�����������⣮

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д�
�����Ŀ
��ͨ��ͭ����ͭ��п��ɣ��㷺���������ġ��ܲĵȣ�Ҳ���������е�����Ϊ�ⶨ��ͭ��ͭ������������ȡ��Ʒ10g�����Ĵ������м���ϡ����ʹ֮��ַ�Ӧ��ʵ�����ݼ�¼���±���
����������ݣ��ش��������⣺
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ����ϡ���������/g | 10 | 10 | 10 | 10 |
| ʣ����������/g | 8.7 | 7.4 | 7 | 7 |
��1����ͭ��ͭ����������Ϊ
��2��ǡ����ȫ��Ӧʱ����Һ�����ʵ����������ǣ�
��Һ�����������ϢϢ��أ���Һ���������ճ�����ͻ�ѧʵ���еij����������±���������Һ�Ͱ�ˮ���ܶ��������ʵ������������ձ���20�棩��
����ϸ������ش��������⣺
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ��� �����������䣩����ˮ���ܶ��� ��������С�䣩
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ 100g������ڡ�С�ڻ���ڣ���
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ����� mL��������0.1����
| ��Һ�����ʵ���������/% | 4 | 12 | 16 | 24 | 28 |
| ������Һ���ܶ�/g/mL | 1.02 | 1.08 | 1.11 | 1.17 | 1.20 |
| ��ˮ���ܶ�/g/mL | 0.98 | 0.95 | 0.94 | 0.91 | 0.90 |
��1��20��ʱ��������Һ�����ʵ���������������������Һ���ܶ���
��2��ȡ12%��������Һ100g���Ƴ�6%����Һ����100g12%��������Һ�м�ˮ������
Ӧ
��3����100g24%�İ�ˮ�м���100gˮ��ҡ�ȣ���Һ�����