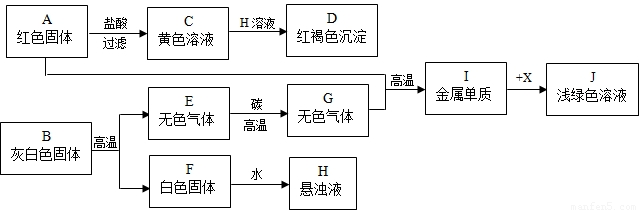
��Ŀ����
��2007?�ߴ��ض�ģ���ҹ���������ȱˮ������Ӱ�����ǵ�����;��÷�չ����ʵʩ��ˮ�������̣���1����ˮ��������ԭ��һ���Ƚ�ˮ���ˮ��ͼ�����ڽ�ˮ��ǵ��ǣ�
��2�����������ۺ�ͨˮ��ij�������ķ�ˮ��dz��ɫ�����ⶨ����һ����������ͭ��ֻ������ˮ�������ۡ������ļ��ɳ�ȥ���е�Cu2+��
��3�������Ȼ�������ˮ�����������������̬���̵�ʵ�ʣ��ٳ�����������ʩ��
A��B��
��4��ˮ����Щ��Ҫ�Ļ�ѧ���ʣ���������д����Ӧ�Ļ�ѧ����ʽ��
A��B��

���𰸡����������������غ㶨�ɿ�����д��ѧ����ʽ����ȷ��ʶ������ˮ����Ҫ�ԣ�
����⣺��1��D�ǽ�ˮ��־�����D��
��2�����������ܺ�ͭ���ӽ�ϳ�������ͭ����������������ƣ�
��3��������ʩ�У�������ˮ���д������ŷţ���ҵ��ˮ���������ŷţ�
��4��ˮ�ܺͶ�����̼��Ӧ����̼�ᣬˮ���������������������ѧ����ʽΪ��CO2+H2O=H2CO3��2H2O 2H2��+O2��
2H2��+O2��
������������Ҫ�����˻�ѧ����ʽ����д���䴦����ˮ�ȷ�������ݣ������������е�֪ʶ���У�
����⣺��1��D�ǽ�ˮ��־�����D��
��2�����������ܺ�ͭ���ӽ�ϳ�������ͭ����������������ƣ�
��3��������ʩ�У�������ˮ���д������ŷţ���ҵ��ˮ���������ŷţ�
��4��ˮ�ܺͶ�����̼��Ӧ����̼�ᣬˮ���������������������ѧ����ʽΪ��CO2+H2O=H2CO3��2H2O
 2H2��+O2��
2H2��+O2��������������Ҫ�����˻�ѧ����ʽ����д���䴦����ˮ�ȷ�������ݣ������������е�֪ʶ���У�

��ϰ��ϵ�д�
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
�����Ŀ
 SO2
SO2 CaO+SO2��
CaO+SO2��