��Ŀ����
��ѧС���ͬѧΪ�˲ⶨijͭ���м�ʽ̼��ͭ[��ѧʽΪCu2��OH��2CO3����Է�������Ϊ222]��������������ȡ��ͭ����Ʒ30g������132.2gϡ����ʱǡ����ȫ��Ӧ��������CO2����4.4g����Ӧ�Ļ�ѧ����ʽ���£�Cu2��OH��2CO3+4HCl=2CuCl2+3H2O+CO2��
������㣺
��1��ͭ����Ʒ�м�ʽ̼��ͭ�������Ƕ��ٿˣ������������Ƕ��٣�
��2����Ӧ��������Һ���������������Ƕ��٣�������ͭ���е����ʲ���ϡ���ᷴӦ��Ҳ������ˮ����
Cu2��OH��2CO3+4HCl=2CuCl2+3H2O+CO2��
222 270 44
x y 4.4g

x=22.2g

y=27g
ͭ����Ʒ�м�ʽ̼��ͭ����������=
 ��100%=74%
��100%=74% ��2����Ӧ�������Ȼ�ͭ��Һ������Ϊ22.2g+132.2g-4.4g=150g
��Ӧ�������Ȼ�ͭ��Һ�����ʵ���������=
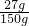 ��100%=18%
��100%=18% �𣺣�1��ͭ����Ʒ�м�ʽ̼��ͭ��������22.2g��������������74%����2����Ӧ�������Ȼ�ͭ��Һ�����ʵ�����������18%��
��������1�����ݼ�ʽ̼��ͭ�����ᷴӦ�Ļ�ѧ����ʽ�����ɵ�������������г�����ʽ�����ɼ�������뷴Ӧ��Cu2��OH��2CO3������������CuCl2��������Ȼ���������������ʽ���㼴�ɣ�
��2����Ӧ����Һ�е�������CuCl2�����ã�2�������������CuCl2������������������������=
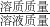 ��100%���㼴�ɣ�
��100%���㼴�ɣ�������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м�����������Ѷ��Դ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�С�մ���Ҫ�ɷ�ΪNaHCO3���г����������Ȼ��ơ���ѧС���ͬѧΪ�˲ⶨС�մ���NaHCO3����������������������ʵ�飺����Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ����õ��й��������±���ʾ��
| �� �� | �� Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� | 9 | 75.4 | 80 |
��1����Ʒ�е�NaHCO3����������
��2��������Һ�����ʵ�����������
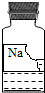
ʵ������һƿ���ܲ������Լ���������ͼ��ʾ�������ȱ�ı�ǩ��ֻʣ�¡�Na���͡�l0%����������֪������ɫҺ�壬�dz��л�ѧ���õ��Լ���
��������⡿��ƿ�Լ�������ʲô��Һ�أ�
���������֡����������ǩ����� �жϣ���ƿ�Լ���������_____������ĸ��ţ���
�жϣ���ƿ�Լ���������_____������ĸ��ţ���
A. �� B�� �� C�� ��
���������ϡ�
�ٳ��л�ѧ�����ĺ��ƻ�������NaCl��NaOH��Na2C03��NHC03��
��Na2C03��NaHC03��Һ���ʼ��� ��
��
�۲ⶨ������(20��)ʱ��4�����ʵ��ܽ�ȵ��������±���ʾ��
��l���ʵ��ܽ��
| ���� | NaCl | NaOH | Na2C03 | NaHC03 |
| �ܽ��/g | 36 | 109 | 215 | 9.6 |
���ó����ۡ�С�������Լ�ƿ��ע��������������10%���ϱ����ܽ�ȵ��������жϣ���ƿ�Լ���������_______��
��������ʵ�顿�ٿ�����NaOH��Һ���ڿ�����Na2C03��Һ�� �ۿ�����NaCl��Һ��
(1 )Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����pH>7����ƿ�Լ���������____��
)Сǿ�ýྻ�IJ�����պȡ����Һ����pH��ֽ�ϣ����pH>7����ƿ�Լ���������____��
(2)СǿΪ��ȷ������Һ�ijɷ֣����ֽ��������2��ʾʵ�顣
��2��ʵ���¼
| �������� | ʵ������ | ���ۼ���ѧ����ʽ |
| ����һ�� ȡ��Ʒ���Թ��У��μ� | �������������� | �������ȷ �������ط�Ӧ�Ļ�ѧ���� ʽ��_______________ |
| ��������Ѳ���������ͨ������ʯ��ˮ�� | ������Һ����� |
����չ��Ӧ�á�(1)����ѡ����Сǿ��ͬ���Լ���ȷ������Һ�ijɷ֣���ѡ��_____��Һ��
(2)С�մ���Ҫ�ɷ�ΪNaHC03���г����������Ȼ��ơ���ѧС���ͬѧΪ�˲ⶨС�մ���NaHC03����������������������ʵ�飺����Ʒ�����ձ��У������������μ�ϡ���ᣬ�����ٲ�������Ϊֹ����õ��й��������±���ʾ��
| �� �� 1 �� Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ����(g) 9 | 75.4 | 80 |
���㣺��Ӧ��������Һ����������������
| �� �� | �� Ʒ | ����ϡ�������� | ��Ӧ����Һ���� |
| ������g�� | 9 | 75.4 | 80 |
��1����Ʒ�е�NaHCO3����������
��2��������Һ�����ʵ�����������