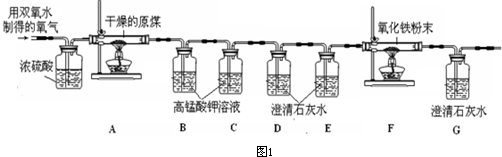
��Ŀ����
Ϊ���Ƴ��л�����������������������½��ʹ����Ȼ�����Ҵ����͵������Դ��
��1������������ȼ�ϵĸ����������£�

�������йؼ���ȼ�ϸ��µ����ɣ���ȷ����______��
A����Ȼ���ǿ���������Դ
B������ȼ�ϵ������ʸ���
C������úȼ�չ����в�������Ⱦ��
����Ȼ������Ҫ�ɷ��Ǽ��飩��Һ��ʯ��������Ҫ�ɷ��DZ���Ͷ��飩��̼Ԫ�ص����������ֱ�ԼΪ75%��82.3%��ͨ�������ݷ�������ͬ�����£�����Ȼ����ȼ�ϱ�Һ��ʯ����������������______��
�ۼ����ڿ�������ȫȼ�յĻ�ѧ����ʽΪ______��
��2�����½����͵�������վ����Ȼ���辭�����ӡ���������ζ������ѹ�ȴ�����������͵�������У��÷��ӵĹ۵������Ȼ����ѹ����������͵�ԭ��______����������������ζ��������������______��
��3��Ϊ��һ�������������������н����ƹ��Ҵ����͵�ʹ�ã���ν�Ҵ��������������м��������Ҵ���϶��ɵ�һ��ȼ�ϣ�
���Ҵ���������______��ѡ����������������
�ڴӻ��������ĽǶȿ�������ȼ�������������______����д��ĸ��ţ���
A��������B��ú��C���Ҵ����ͣ�D��ú������Ҫ��CO����
��1������������ȼ�ϵĸ����������£�

�������йؼ���ȼ�ϸ��µ����ɣ���ȷ����______��
A����Ȼ���ǿ���������Դ
B������ȼ�ϵ������ʸ���
C������úȼ�չ����в�������Ⱦ��
����Ȼ������Ҫ�ɷ��Ǽ��飩��Һ��ʯ��������Ҫ�ɷ��DZ���Ͷ��飩��̼Ԫ�ص����������ֱ�ԼΪ75%��82.3%��ͨ�������ݷ�������ͬ�����£�����Ȼ����ȼ�ϱ�Һ��ʯ����������������______��
�ۼ����ڿ�������ȫȼ�յĻ�ѧ����ʽΪ______��
��2�����½����͵�������վ����Ȼ���辭�����ӡ���������ζ������ѹ�ȴ�����������͵�������У��÷��ӵĹ۵������Ȼ����ѹ����������͵�ԭ��______����������������ζ��������������______��
��3��Ϊ��һ�������������������н����ƹ��Ҵ����͵�ʹ�ã���ν�Ҵ��������������м��������Ҵ���϶��ɵ�һ��ȼ�ϣ�
���Ҵ���������______��ѡ����������������
�ڴӻ��������ĽǶȿ�������ȼ�������������______����д��ĸ��ţ���
A��������B��ú��C���Ҵ����ͣ�D��ú������Ҫ��CO����
��1������Ȼ���Dz�����������Դ�����B��C
����Ȼ����Һ��ʯ�����ĺ�̼���ͣ�ȼ��ʱ�����Ķ�����̼��һ����̼���٣�
�����ͬ������ȼ��ʱ������һ����̼�Ͷ�����̼���٣�
�ۼ���ȼ�յĻ�ѧ����ʽΪ��CH4+2O2
CO2+2H2O��
��2������֮����һ���ļ����ѹǿ��ʱ���С��ѹǿСʱ����������֮���м����
��������������ζ������֪����Ȼ��й¶ʱ�����������ܹ�֪����Ȼ���Ƿ�й¶����ֹ��ը��
��3�����Ҵ����������Ҵ���������ɵģ����ڻ����������
������ȼ�յIJ�����ˮ������Ⱦ���������A
����Ȼ����Һ��ʯ�����ĺ�̼���ͣ�ȼ��ʱ�����Ķ�����̼��һ����̼���٣�
�����ͬ������ȼ��ʱ������һ����̼�Ͷ�����̼���٣�
�ۼ���ȼ�յĻ�ѧ����ʽΪ��CH4+2O2
| ||
��2������֮����һ���ļ����ѹǿ��ʱ���С��ѹǿСʱ����������֮���м����
��������������ζ������֪����Ȼ��й¶ʱ�����������ܹ�֪����Ȼ���Ƿ�й¶����ֹ��ը��
��3�����Ҵ����������Ҵ���������ɵģ����ڻ����������
������ȼ�յIJ�����ˮ������Ⱦ���������A

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ