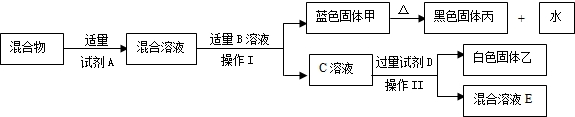
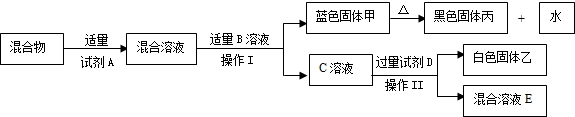
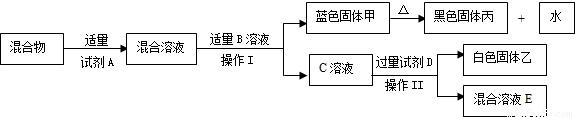
��Ŀ����
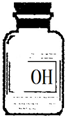 С���ڰ���ʦ����ʵ����ʱ��������ҩƷ���з�����һƿ��ǩֻʣ�¡�OH�������İ�ɫ���壨��ͼ��ʾ����������С������ƿ��ɫ������е�̽�����̣�
С���ڰ���ʦ����ʵ����ʱ��������ҩƷ���з�����һƿ��ǩֻʣ�¡�OH�������İ�ɫ���壨��ͼ��ʾ����������С������ƿ��ɫ������е�̽�����̣�������룺
��1�����ݱ�ǩ�����Ļ�ѧʽ�жϣ���ƿ�Լ�����
��
��
��ѡ��ᡱ��������Ρ�������2����֪���ֽ���������������ʱ�������Ļ�����ָ����������ɫ�����±���
| ��������Ԫ�� | �� | �� | �� | ͭ |
| ������ɫ | ש��ɫ | ��ɫ | ��ɫ������ɫ�ܲ���Ƭ�� | ��ɫ |
��������
��������
����3��С��������ƿ�Լ����ܱ��ʣ����û�ѧ����ʽ��ʾС����������ݣ�
2NaOH+CO2�TNa2CO3+H20
2NaOH+CO2�TNa2CO3+H20
����4��������ʵ���С���IJ��������֤��
| ʵ�鲽�� | ʵ������ | ʵ����� |
ȡ������Ʒ���Թ��в��μ�ϡ���� ȡ������Ʒ���Թ��в��μ�ϡ���� |
�����ݲ��� �����ݲ��� |
��ƿ�Լ��Ѿ����ʣ����ʵ����������������Լ�������Ӧ�Ļ�ѧ����ʽ�� Na2CO3+2HCl=2NaCl+H2O+CO2�� Na2CO3+2HCl=2NaCl+H2O+CO2�� �� |
����������Һ
����������Һ
��ѡ�ϡ���ᡱ�����Ȼ�����Һ��������������Һ�������Ʊ������漰��Ӧ�Ļ�ѧ����ʽ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH
Na2CO3+Ca��OH��2=CaCO3��+2NaOH
����������1����������εĶ���ɷ������ʵ����ࣻ
��2��������ɫ��Ӧ����Ϣ�Կ��ܵ����ʽ��з����ƶϼ��ɣ�
��3���������Ƽ��ܹ����տ����е�ˮ�֣�Ҳ�ܺͿ����еĶ�����̼��Ӧ����̼���ƣ�
��4���������Ƽ��ܹ����տ����е�ˮ�֣�Ҳ�ܺͿ����еĶ�����̼��Ӧ����̼���ƣ������֤���������Ƿ���ʣ�ֻҪ֤��̼���ƵĴ��ڼ��ɣ�
��5������̼���ƺ���������������̼��ƺ��������Ƶ�֪ʶ���������
��2��������ɫ��Ӧ����Ϣ�Կ��ܵ����ʽ��з����ƶϼ��ɣ�
��3���������Ƽ��ܹ����տ����е�ˮ�֣�Ҳ�ܺͿ����еĶ�����̼��Ӧ����̼���ƣ�
��4���������Ƽ��ܹ����տ����е�ˮ�֣�Ҳ�ܺͿ����еĶ�����̼��Ӧ����̼���ƣ������֤���������Ƿ���ʣ�ֻҪ֤��̼���ƵĴ��ڼ��ɣ�
��5������̼���ƺ���������������̼��ƺ��������Ƶ�֪ʶ���������
����⣺��1��������εĶ����ֻ֪�м������ʻ��ʽ�δ��������������������ڱ�ǩ�����Ҳ࣬���Բ����Ǽ�ʽ�Σ�ֻ��Ϊ�
��2��������ɫ��Ӧ����Ϣ�۲쵽����ʻ�ɫ��˵�����������ӣ����Կɲ�����������������ƣ�
��3�����������ڿ������ܺͿ����еĶ�����̼��Ӧ����̼���ƺ�ˮ�����ʣ��䷽��ʽΪ2NaOH+CO2�TNa2CO3+H20��
��4���������Ƽ��ܹ����տ����е�ˮ�֣�Ҳ�ܺͿ����еĶ�����̼��Ӧ����̼���ƣ������֤���������Ƿ���ʣ�ֻҪ֤��̼���ƵĴ��ڼ��ɣ���˼������ῴ�Ƿ��������������Ǽ������������Ƿ���ʵ�ԭ���漰���ķ���ʽΪNa2CO3+2HCl=2NaCl+H2O+CO2����
��5����Ϊ̼���ƺ���������������̼��ƺ�������������Ӧѡ�õ��Լ�������������Һ���䷽��ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
�ʴ�Ϊ����1�����2���������ƣ���3��2NaOH+CO2�TNa2CO3+H20��
��4��
��5������������Һ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
��2��������ɫ��Ӧ����Ϣ�۲쵽����ʻ�ɫ��˵�����������ӣ����Կɲ�����������������ƣ�
��3�����������ڿ������ܺͿ����еĶ�����̼��Ӧ����̼���ƺ�ˮ�����ʣ��䷽��ʽΪ2NaOH+CO2�TNa2CO3+H20��
��4���������Ƽ��ܹ����տ����е�ˮ�֣�Ҳ�ܺͿ����еĶ�����̼��Ӧ����̼���ƣ������֤���������Ƿ���ʣ�ֻҪ֤��̼���ƵĴ��ڼ��ɣ���˼������ῴ�Ƿ��������������Ǽ������������Ƿ���ʵ�ԭ���漰���ķ���ʽΪNa2CO3+2HCl=2NaCl+H2O+CO2����
��5����Ϊ̼���ƺ���������������̼��ƺ�������������Ӧѡ�õ��Լ�������������Һ���䷽��ʽΪ��Na2CO3+Ca��OH��2=CaCO3��+2NaOH��
�ʴ�Ϊ����1�����2���������ƣ���3��2NaOH+CO2�TNa2CO3+H20��
��4��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ȡ������Ʒ���Թ��в��μ�ϡ���� | �����ݲ��� | ��ƿ�Լ��Ѿ����ʣ����ʵ����������������Լ�������Ӧ�Ļ�ѧ����ʽ�� Na2CO3+2HCl=2NaCl+H2O+CO2���� |
�����������Ƕ��������ƹ�����ʵ����֪ʶ�Ŀ��飬���м��黯ѧҩƷ�Ƿ���ʵ�һ�㷽���ǣ�Ū������ʱ��ʺ�����ʲô��Ȼ�����������ѡ����Ӧ��ҩƷ���飮ע�����ʱһ��Ҫ�����Ե�����

��ϰ��ϵ�д�
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
�����Ŀ
С���ڰ���ʦ����ʵ����ʱ��������ҩƷ���з�����һƿ��ǩֻʣ�¡�OH�������İ�ɫ���壨��ͼ��ʾ����������С������ƿ��ɫ������е�̽�����̣�
������룺
��1�����ݱ�ǩ�����Ļ�ѧʽ�жϣ���ƿ�Լ�����______��ѡ��ᡱ��������Ρ�����
��2����֪���ֽ���������������ʱ�������Ļ�����ָ����������ɫ�����±���
С��ȡ��ƿ�Լ�����Ʒ�ڻ��������գ��۲쵽����ʻ�ɫ������Ϊ�����Լ�ƿ��ǩ�ϵĻ�ѧʽ��______��
��3��С��������ƿ�Լ����ܱ��ʣ����û�ѧ����ʽ��ʾС����������ݣ�______��
��4��������ʵ���С���IJ��������֤��
��5��С����������ƿ���ʵ��Լ�ͨ��һ����Ӧ�Ʊ���ǩ�ϵ����ʣ�����ΪӦѡ����Լ���______��ѡ�ϡ���ᡱ�����Ȼ�����Һ��������������Һ�������Ʊ������漰��Ӧ�Ļ�ѧ����ʽ��______��
������룺
��1�����ݱ�ǩ�����Ļ�ѧʽ�жϣ���ƿ�Լ�����______��ѡ��ᡱ��������Ρ�����
��2����֪���ֽ���������������ʱ�������Ļ�����ָ����������ɫ�����±���
| ��������Ԫ�� | �� | �� | �� | ͭ |
| ������ɫ | ש��ɫ | ��ɫ | ��ɫ������ɫ�ܲ���Ƭ�� | ��ɫ |
��3��С��������ƿ�Լ����ܱ��ʣ����û�ѧ����ʽ��ʾС����������ݣ�______��
��4��������ʵ���С���IJ��������֤��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ______ | ______ | ��ƿ�Լ��Ѿ����ʣ����ʵ����������������Լ�������Ӧ�Ļ�ѧ����ʽ�� ______�� |