��Ŀ����
A��B��C��D��E�dz��л�ѧ�е����ֳ������ʣ�C��D�ǵ��ʣ�CΪ�Ϻ�ɫ���壬D���ܶ���С�����壬B��Ũ��Һ���������������֪Fe��A��B��ˮ��Һ�ֱ��ܷ�����Ӧ��
��Fe+A��C+E����Fe+B��D+E
��1��д��A��B��C�Ļ�ѧʽ��A
��2������Fe��A��C���ֹ�����ɵĻ���ij��ѧ��ȤС���ͬѧ��ͨ��ʵ��ⶨ�û������C���ʵ�����������
�����ʵ�顿��ͼ��ʾ
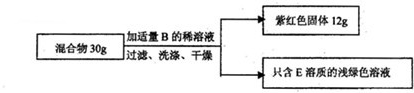
�����ݴ�����
��С�ո������ݼ�������������C���ʵ���������Ϊ��
��100%��Сǿ��Ϊ���ݴ�������ȷ��ԭ����
�����Ǿ�������������ֻ�ȱ�ٱ�Ҫ��ʵ�����ݣ������������飬������ƽ�Ƴ���Ӧǰ��������������0��1g���Ӷ�����������C���ʵ���������Ϊ
��ʵ�鷴˼��
ͬѧ�ǶԸû����ijɷ־�������������֣�ֻ�������ʵ���������Ҳ�ܲⶨC���ʵ�������������д�����������鷽��
��Fe+A��C+E����Fe+B��D+E
��1��д��A��B��C�Ļ�ѧʽ��A
CuSO4
CuSO4
BH2SO4
H2SO4
CCu
Cu
��2������Fe��A��C���ֹ�����ɵĻ���ij��ѧ��ȤС���ͬѧ��ͨ��ʵ��ⶨ�û������C���ʵ�����������
�����ʵ�顿��ͼ��ʾ

�����ݴ�����
��С�ո������ݼ�������������C���ʵ���������Ϊ��
| 12g | 30g |
12g�Ϻ�ɫ�����а�������������ͭ��Ӧ���ɵ�ͭ����������ͭ��Ӧ������ͭ��12g�Ϻ�ɫ���壬��ֻ��ԭ����C���ʣ���12g�Ϻ�ɫ�������������ԭ��C���ʵ���������
12g�Ϻ�ɫ�����а�������������ͭ��Ӧ���ɵ�ͭ����������ͭ��Ӧ������ͭ��12g�Ϻ�ɫ���壬��ֻ��ԭ����C���ʣ���12g�Ϻ�ɫ�������������ԭ��C���ʵ���������
�����Ǿ�������������ֻ�ȱ�ٱ�Ҫ��ʵ�����ݣ������������飬������ƽ�Ƴ���Ӧǰ��������������0��1g���Ӷ�����������C���ʵ���������Ϊ
18.7%
18.7%
����ʵ�鷴˼��
ͬѧ�ǶԸû����ijɷ־�������������֣�ֻ�������ʵ���������Ҳ�ܲⶨC���ʵ�������������д�����������鷽��
����������ˮ�����ˣ�ϴ�Ӻ�ɣ��Ƶ������������ô��������������Ƶ�ʣ�����ʵ�������ͭ����������ͭ��������������������ͭ������������
����������ˮ�����ˣ�ϴ�Ӻ�ɣ��Ƶ������������ô��������������Ƶ�ʣ�����ʵ�������ͭ����������ͭ��������������������ͭ������������
����������1������ȷ����⣬��֪��������塢Ũ��Һ����������������ʡ��Ϻ�ɫ����ֱ���ʲô��Ȼ�������֪������ͼʾ�����������ķ�Ӧ��ʼ�ƶϣ�������룬�Ϳ���ȷ���
��2���ٸ��ݻ�ѧ��Ӧ�����ʵı仯������з������ɣ����������ᷴӦ�������������ٵ�����Ϊ������������������������������ᷴӦ������������������ͭ������Ϊy��������������ͭ��Ӧ�Ļ�ѧ����ʽ������ͭ������ʾ�������������ٸ��ݷ�Ӧ��ͭ��������12�˼�Ϊԭ�������ͭ������������ͭ��ͭԪ������֮�ͣ�
��2���ٸ��ݻ�ѧ��Ӧ�����ʵı仯������з������ɣ����������ᷴӦ�������������ٵ�����Ϊ������������������������������ᷴӦ������������������ͭ������Ϊy��������������ͭ��Ӧ�Ļ�ѧ����ʽ������ͭ������ʾ�������������ٸ��ݷ�Ӧ��ͭ��������12�˼�Ϊԭ�������ͭ������������ͭ��ͭԪ������֮�ͣ�
����⣺��1���ɡ�C��D�ǵ��ʣ�CΪ�Ϻ�ɫ���塱�����ƶϣ�CΪCu��D���ܶ���С������ʿ��ƶ�D��H2���ɡ�B��Ũ��Һ����������������ж�B��Ũ����ʢ�Fe+B��D+E�Ļ�ѧ����ʽΪ��Fe+H2SO4=FeSO4+H2�����ʿ��ƶϣ�EΪFeSO4��
��ô��Fe+A��C+E�Ļ�ѧ����ʽΪ��Fe+CuSO4=FeSO4+Cu���ʿ��ƶ�AΪCuSO4��
��ѡCuSO4��H2SO4��Cu��
��2���ٻ�����м����������ᣬ��������ͭ��Ӧ��������������ͭ��12g�Ϻ�ɫ�����а�������������ͭ��Ӧ���ɵ�ͭ����������ͭ��Ӧ������ͭ��12g�Ϻ�ɫ���壬��ֻ��ԭ����C���ʣ���12g�Ϻ�ɫ�������������ԭ��C���ʵ���������
�ڼ��ٵ�����Ϊ����������������������Ϊ0.2g���������ᷴӦ����������Ϊx
Fe+H2SO4 �TFeSO4+H2��
56 2
x 0.1g
=
x=2.8g
������������ͭ������Ϊy��������ͭ��Ӧ����������Ϊz
Fe+CuSO4�TCu+FeSO4
56����160
z������y
=
z=
=
����ͭ��ͭԪ�ص�����Ϊy��
=
��Ӧ��ͭ������Ϊ30g-2.8g-
-x+
=12g x=16
ԭ�������ͭ������Ϊ30g-2.8g-
��16-16=5.6g
����ԭ�������ͭ����������Ϊ
��100%=18.7%
���ܱ�����������ͭ������ˮ������ͭ������ˮ����˿ɽ���������ˮ�����ˣ�ϴ�Ӻ�ɣ��Ƶ���������������Ϊ����ͭ�Ļ����ô���������������ȡ������������ͭ����������ͭ��������������������ͭ������������
�ʴ�Ϊ����1��CuSO4B H2SO4C Cu
��2����12g�Ϻ�ɫ�����а�������������ͭ��Ӧ���ɵ�ͭ����������ͭ��Ӧ������ͭ��12g�Ϻ�ɫ���壬��ֻ��ԭ����C���ʣ���12g�Ϻ�ɫ�������������ԭ��C���ʵ���������
��18.7g
����������ˮ�����ˣ�ϴ�Ӻ�ɣ��Ƶ������������ô��������������Ƶ�ʣ�����ʵ�������ͭ����������ͭ��������������������ͭ������������
��ô��Fe+A��C+E�Ļ�ѧ����ʽΪ��Fe+CuSO4=FeSO4+Cu���ʿ��ƶ�AΪCuSO4��
��ѡCuSO4��H2SO4��Cu��
��2���ٻ�����м����������ᣬ��������ͭ��Ӧ��������������ͭ��12g�Ϻ�ɫ�����а�������������ͭ��Ӧ���ɵ�ͭ����������ͭ��Ӧ������ͭ��12g�Ϻ�ɫ���壬��ֻ��ԭ����C���ʣ���12g�Ϻ�ɫ�������������ԭ��C���ʵ���������
�ڼ��ٵ�����Ϊ����������������������Ϊ0.2g���������ᷴӦ����������Ϊx
Fe+H2SO4 �TFeSO4+H2��
56 2
x 0.1g
| 56 |
| x |
| 2 |
| 0.1g |
������������ͭ������Ϊy��������ͭ��Ӧ����������Ϊz
Fe+CuSO4�TCu+FeSO4
56����160
z������y
| 56 |
| z |
| 160 |
| y |
z=
| 56y |
| 160 |
| 7y |
| 20 |
����ͭ��ͭԪ�ص�����Ϊy��
| 64 |
| 160 |
| 8y |
| 20 |
��Ӧ��ͭ������Ϊ30g-2.8g-
| 7x |
| 20 |
| 8x |
| 20 |
ԭ�������ͭ������Ϊ30g-2.8g-
| 7 |
| 20 |
����ԭ�������ͭ����������Ϊ
| 5.6g |
| 30g |
���ܱ�����������ͭ������ˮ������ͭ������ˮ����˿ɽ���������ˮ�����ˣ�ϴ�Ӻ�ɣ��Ƶ���������������Ϊ����ͭ�Ļ����ô���������������ȡ������������ͭ����������ͭ��������������������ͭ������������
�ʴ�Ϊ����1��CuSO4B H2SO4C Cu
��2����12g�Ϻ�ɫ�����а�������������ͭ��Ӧ���ɵ�ͭ����������ͭ��Ӧ������ͭ��12g�Ϻ�ɫ���壬��ֻ��ԭ����C���ʣ���12g�Ϻ�ɫ�������������ԭ��C���ʵ���������
��18.7g
����������ˮ�����ˣ�ϴ�Ӻ�ɣ��Ƶ������������ô��������������Ƶ�ʣ�����ʵ�������ͭ����������ͭ��������������������ͭ������������
������������Ҫ����ѧ����һЩ�������ʵ����ú����ʵ���ʶ���Լ����û�ѧ����ʽ�������������м�����жϵ�������

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��֪A��B��C��D��E�dz��л�ѧ�������������ʣ�����A��D�Ǻ�ɫ���壬AΪ��ɫ���嵥�ʣ�DΪ��ɫ��������������к�������ʹ����㷺�Ľ���Ԫ�أ�B��C��E����ɫ���壬��������һ�������µ�ת����ϵ����ͼ��ʾ��������Ӧ�������������ȥ����
��֪A��B��C��D��E�dz��л�ѧ�������������ʣ�����A��D�Ǻ�ɫ���壬AΪ��ɫ���嵥�ʣ�DΪ��ɫ��������������к�������ʹ����㷺�Ľ���Ԫ�أ�B��C��E����ɫ���壬��������һ�������µ�ת����ϵ����ͼ��ʾ��������Ӧ�������������ȥ���� 23����֪A��B��C��D��E�dz��л�ѧ�ﳣ�����������ʣ�������һ���������ܷ�����ͼ��ʾ��ת�������з�Ӧ���Ǹ��ֽⷴӦ��E���������ЧӦ����Ҫ���壮
23����֪A��B��C��D��E�dz��л�ѧ�ﳣ�����������ʣ�������һ���������ܷ�����ͼ��ʾ��ת�������з�Ӧ���Ǹ��ֽⷴӦ��E���������ЧӦ����Ҫ���壮 A��B��C��D��E�dz������ʣ�����B��E�ǿ������������壬C��������Ҫ�ɷ֣���ͼ��ʾ��ֱ�߱�ʾ����ܹ���Ӧ����ͷ��ʾת���ķ���
A��B��C��D��E�dz������ʣ�����B��E�ǿ������������壬C��������Ҫ�ɷ֣���ͼ��ʾ��ֱ�߱�ʾ����ܹ���Ӧ����ͷ��ʾת���ķ��� ��2013?���ݶ�ģ��A��B��C��D��E�dz��л�ѧ�г����IJ�ͬ�������ʣ����ʰ����ʡ�������ᡢ��η��ࣩ����֪A�ǵ��ʣ�C�Ǻ���ɫ���壻E��ˮ��Һ��ʹ��̪��Һ��Ϊ��ɫ���Σ�ͼ�С�-����ʾ��������������֮����Է�����Ӧ����������ʾ��ijһ���ʿ��Ƶ���һ���ʣ����ַ�Ӧ������P��Ӧ��������ȥ�����ش��������⣺
��2013?���ݶ�ģ��A��B��C��D��E�dz��л�ѧ�г����IJ�ͬ�������ʣ����ʰ����ʡ�������ᡢ��η��ࣩ����֪A�ǵ��ʣ�C�Ǻ���ɫ���壻E��ˮ��Һ��ʹ��̪��Һ��Ϊ��ɫ���Σ�ͼ�С�-����ʾ��������������֮����Է�����Ӧ����������ʾ��ijһ���ʿ��Ƶ���һ���ʣ����ַ�Ӧ������P��Ӧ��������ȥ�����ش��������⣺ A��B��C��D��E�dz��л�ѧ�г�����5�����ʣ����Ƕ�����һ����ͬ��Ԫ�أ���ͼ��ʾ������֮���ת����ϵ�����У�AΪʳ�ε���Ҫ�ɷ֣�B�к���Ԫ�أ�D��E����Һ������ɫ��
A��B��C��D��E�dz��л�ѧ�г�����5�����ʣ����Ƕ�����һ����ͬ��Ԫ�أ���ͼ��ʾ������֮���ת����ϵ�����У�AΪʳ�ε���Ҫ�ɷ֣�B�к���Ԫ�أ�D��E����Һ������ɫ��