��Ŀ����
����Ŀ��ˮ������֮Դ
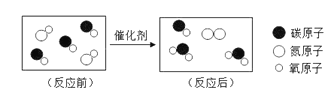
��1��1��ˮ����20��ˮΪ1mL�ƣ��д�Լ����1.67��1023��ˮ________���á����ӡ�����ԭ�ӡ������ӡ���ա���ˮ���⡢��Ԫ�ص�������Ϊ_______________��
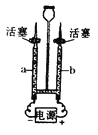
��2����ͼΪ���ˮ��ʵ��װ��ͼ��ͨ��һ��ʱ�������a ���ռ���������Ϊ_______���ѧʽ����������Dz�����b �������_________�������ˮ�Ļ�ѧ����ʽΪ_______________��
��3����ͼ�ǿ�ѧ���о�������һ����̫����Ϊ��Դ�ĵ���ѭ���ֽ�ˮ����ķ�Ӧ����ͼ������ͼʾ�ش�
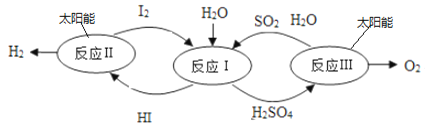
�� HI ����______________��
�ڡ���ӦI���Ļ�ѧ����ʽΪ_____________��
�۾����������̣�ÿ�ֽ�a gˮ�ܵõ�����__________ g��
���𰸡����� 1��8 H2 2 2H2O![]() 2H2��+O2�� �⻯�� I2+2H2O+SO2=2HI+H2SO4
2H2��+O2�� �⻯�� I2+2H2O+SO2=2HI+H2SO4 ![]()
��������
��1��1��ˮ����20��ˮΪ1mL�ƣ��д�Լ��1.67��1023��ˮ���ӣ�ˮ���⡢��Ԫ�ص�������Ϊ��1��2����16=1��8��
��2����ͼΪ���ˮ��ʵ��װ��ͼ�����ݵ��ˮʵ��������Ϊ���������������һ������֪ͨ��һ��ʱ�������a���ռ���������࣬ΪH2��������Dz�����b������� 2�������ˮ�Ļ�ѧ����ʽΪ2H2O![]() 2H2��+O2����
2H2��+O2����
��3����HI����Ԫ����ɵĻ��������Ӧ���ǴӺ���ǰ�����м�Ӹ����������⻯�⣻
������Ӧ�����ǵ��ˮ�Լ������������ɵ⻯���Լ����ᣬ��Ӧ�Ļ�ѧ����ʽΪ I2+2H2O+SO2=2HI+H2SO4��
�������������̣�ÿ�ֽ�agˮ�ܵõ�����������Ϊag��![]() =
=![]() g��
g��

 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�����Ŀ���������������������������ϢϢ��ء�ѧϰ�������������ʺͽ����Ļ��˳���ܰ������Ƿ��ֺ��о�����Ľ�����
��1������ϰ���ϳ�Ϊ����𡱵Ľ�����ͭ���������У��ѽ���______����Ԫ�ط��ţ���λ���Ƶ�������÷��Ͻ��������_____���ǿ������������ǿ������˳��
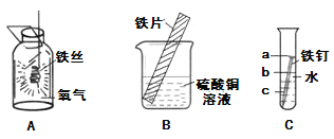
��2��������ͼ�ش��й����⣺
��д��ʵ��A�д��ڵ�һ�����Դ���__________��
��ʵ��B�пɹ۲쵽��Ƭ�ı���_________����
��ʵ��C�пɹ۲쵽�Թ������������������______�������a����b����c����
��3����ν�Ͻ𣬾��Dz�ͬ�ֽ������������ǽ��������ۻ�״̬���γɵ�һ���ۺ��
Na | Cu | Al | Fe | |
�۵㣨�棩 | 97��5 | 1083 | 660 | 1535 |
�е㣨�棩 | 883 | 2595 | 2200 | 3000 |
�����ϱ������ֽ������۷е㣬�ж����в����γɺϽ����_______��
a��Cu��Al b��Fe��Cu c��Fe��Na
����Ŀ����һ�������£���һ���ܱ������ڷ���ij��Ӧ����÷�Ӧǰ������ʵ��������±���ʾ������˵���������(����)
�� �� | �� �� | ������̼ | ˮ���� | W |
��Ӧǰ����/g | 50 | 1 | 1 | 23 |
��Ӧ������/g | 2 | 45 | 28 | x |
A. ���������غ㶨�ɣ�x��ֵӦΪ0 B. ��Ӧ����������Ƕ�����̼��ˮ
C. ����W��̼���⡢������Ԫ�� D. ����Wֻ��̼��������Ԫ��