��Ŀ����
�ش��������⣬д���йػ�ѧ����ʽ��
��1��������ͭ��Һ�м����Ȼ�����Һ���۲쵽�������� ����Ӧ�Ļ�ѧ����ʽΪ ��
��2��þ�ڿ�����ȼ����������þ�͵���þ�����е�Ϊ��3�ۣ�������þ��ˮ��Ӧ����������þ�Ͱ�����NH3����
��д������þ�Ļ�ѧʽ ��
����֪����þ��һ�ֻ���ɫ�Ĺ��壮����þ�ڿ�����ȼ�յ�ʵ������֪�������������£�þ����������е� ���ϣ������� ��
��д������þ��ˮ��Ӧ�Ļ�ѧ����ʽ ��
�� g����þ�к��� 24g þԪ�أ���ȷ�� 0.1g����
��1��CuSO4+BaCl2��BaSO4��+CuCl2����2����Mg3N2����O2��������������ǰ�ɫ�ģ�˵��������MgO�϶࣬Mg3N2���٣���Mg3N2+6H2O��3Mg(OH)2��+2NH3������33.3g
���������������1������ͭ���Ȼ�����Ӧ�������ᱵ�������Ȼ�ͭ���ݴ��жϷ�Ӧ�������д����ʽ������ͭ���Ȼ�����Ӧ�������ᱵ�������Ȼ�ͭ�����Զ���Ϻ��۲쵽�а�ɫ�������ɣ�����ʽ�ǣ�CuSO4+BaCl2��BaSO4��+CuCl2��
��2���پݻ�ѧʽ����д���н�𡣵���þ�е�Ϊ��3�ۣ���Ϊ��2�ۣ���������ǰ�����ۺ��ݽ��淨��д���ʵĻ�ѧʽΪ��Mg3N2��
��þ�ڿ�����ȼ��ʱ������������ǰ�ɫ�ģ��ݴ˷������þ�ڿ�����ȼ��ʱ������������ǰ�ɫ�ģ�������þ��һ�ֻ���ɫ�Ĺ��壬����þ�ڿ�����ȼ��ʱ������������Ӧ��
�۾ݷ�Ӧ���������д����ʽ����ע����ƽ������þ��ˮ��Ӧ����������þ�Ͱ�����NH3��������ʽ�ǣ�Mg3N2+6H2O��3Mg(OH)2��+2NH3����
�ܾݻ�������Ԫ�ص������������м��㡣���ݡ�������ijԪ�ص��������û�������������û������и�Ԫ�ص�������������֪������þ�������ǣ�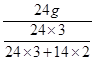 ��33.3g.
��33.3g.
���㣺�����εĻ�ѧ���ʣ���ѧʽ����д�����壻��������ijԪ�ص��������㣻��ѧ����ʽ��д

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߡ���ͼ��Ԫ�����ڱ���һ���֣�
| �� ���� | ��A | | 0 | |||||
| 1 | 1 H 1.008 | ��A | ��A | ��A | ��A | ��A | ��A | 2 He 4.003 |
| 2 | 3 Li 6.941 | 4 Be 9.012 | 5 B 10.8l | 6 C 12.01 | 7 N 14.0l | 8 O 16.00 | 9 F 19.00 | 10 Ne 20.8l |
| 3 | 11 Na 22.99 | 12 Mg 24.31 | 13 Al 26.98 | 14 Si 28.09 | 15 P 30.97 | 16 S 32.06 | 17 Cl 35.45 | 18 Ar 39.95 |
��2�����в�ͬ��Ԫ����ʵ������� (�����)��
A�� ��������ͬ B�� ��������ͬ C�� ���ԭ��������ͬ D�� ��������ͬ
��3�����գ���ѧ�������˹��ϳɵ�118��Ԫ�أ���Ԫ�صĺ˵����Ϊ ��
��4���ӱ���ѡ���ʵ���Ԫ�ط�����գ�
�ؿ��к������Ľ���Ԫ�� �������к����ڶ���Ԫ�� ��
�����к�������Ԫ�� �������к�������Ԫ�� ��
��5������1��8��Ԫ�ؿ���ɵ�������Ļ�ѧʽΪ �� ��