��Ŀ����
��������a��b��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ʒ֣�ʵ����Ҫ����50g������������Ϊ20%���������Һ������25g������������Ϊ40%���������Һ��20g������������Ϊ15%���������Һ���㹻�������ؾ��������ˮ����ѡ��������ҩƷ������������Ʒ��������±���
| ���Ʒ�����ֻҪ˵������ʱ����ĸ���ҩƷ���������ɣ� | |
| ����һ | ______ |
| ������ | ______ |
| ������ | ______ |

ʯ��ʯ��Ʒ�е����ʲ������ᷴӦ���Ҳ�����ϡ���ᣬ������ͼ�龰��дһ�������⣬Ҫ��3�����⣬��д��������̣�
���𰸡�������a������������������Ϊ20%���������Һ���ɲ�ȡ�������ַ�����һ��������ع����ˮ�ܽ�����Ƴ�20%���������Һ������ʹ��40%�������Ũ��Һ��ˮϡ�����������20%���������Һ����������ʹ��15%���������Һ����������ع����ˮ�������20%���������Һ���������������ݼ��������Ҫ�������ʵ����������ʵ�鷽������ƣ�
b���ڱ�����Ŀ������������������Ϣ�������ڱ���ʱ����Ҫд������̣����Ա��Ƶ���Ŀһ���Լ��ܼܺ���������
����⣺����һ������粒����ˮ�ܽ�����50g���ʵ���������Ϊ20%���������Һ��
����淋�����=50g×20%=10g��ˮ����Ϊ50g-10g=40g����40mL��
��������40%�������Ũ��Һ��ˮϡ������50g���ʵ���������Ϊ20%���������Һ��
�ⷨһ�����飩����Һ��ˮ�������������������Сһ�롱��ȡ25g40%�������Ũ��Һ�� 25g����25mL��ˮ��Ͼ��ȣ�
�ⷨ����������50g���ʵ���������Ϊ20%���������Һ��Ҫ40%���������Һ����Ϊx
50g×20%=x×40%
��֮�� x=25g�����ṩ25g40%���������Һ����ǡ���������Ҫ��
��Ҫ��ˮ����Ϊ50g-25g=25g����25mL��
��������20g15%���������Һ����������粒����ˮ�������50g���ʵ���������Ϊ20%���������Һ��
50g���ʵ���������Ϊ20%���������Һ�к����������=50g×20%=10g
��20g15%���������Һ�к�������淋�����=20g×15%=3g
���Ի�Ӧ��������淋�����=10g-3g=7g
����Ҫˮ����Ϊ50g-20g-7g=23g����23mL����
�ʴ�Ϊ��
����һ��10gKNO3�����40gˮ��
��������25g40%��KNO3��Һ��25gˮ��
��������20g15%��KNO3��Һ��7gKNO3�����23gˮ��
b�����⣺ȡ12.5gʯ��ʯ��Ʒ��100g������ϡ���ᷴӦ��ֺ�������������Ϊ108.1g���ٲ���������̼������Ϊ���٣�����Ʒ��̼�����������Ϊ���٣��۷�Ӧ�����������ʵ����������Ƕ��٣�
�⣺�����ɵ�m��CO2��=12.5g+100g-108.1g=4.4g
����̼��Ƶ�����Ϊx����������������Ϊy�������Ȼ�������Ϊz
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
x y z 4.4g
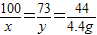
x=10g��y=7.3g��z=11.1g
��Ʒ��̼�����������= =80%
=80%
�۷�Ӧ�����������ʵ���������= =51%
=51%
�𣺢ٲ���������̼������Ϊ4.4g������Ʒ��̼�����������Ϊ80%���۷�Ӧ�����������ʵ�����������51%����ֻҪ���3��С�Ⲣ��Լ��ɣ�������������ⷨ���ɣ���
������������к�ǿ�Ŀ����ԣ����Բ�ȡ���ַ���������Һ�����ơ�����������ұ�д����ַ���ѧ���Ĵ����ԣ�
b���ڱ�����Ŀ������������������Ϣ�������ڱ���ʱ����Ҫд������̣����Ա��Ƶ���Ŀһ���Լ��ܼܺ���������
����⣺����һ������粒����ˮ�ܽ�����50g���ʵ���������Ϊ20%���������Һ��
����淋�����=50g×20%=10g��ˮ����Ϊ50g-10g=40g����40mL��
��������40%�������Ũ��Һ��ˮϡ������50g���ʵ���������Ϊ20%���������Һ��
�ⷨһ�����飩����Һ��ˮ�������������������Сһ�롱��ȡ25g40%�������Ũ��Һ�� 25g����25mL��ˮ��Ͼ��ȣ�
�ⷨ����������50g���ʵ���������Ϊ20%���������Һ��Ҫ40%���������Һ����Ϊx
50g×20%=x×40%
��֮�� x=25g�����ṩ25g40%���������Һ����ǡ���������Ҫ��
��Ҫ��ˮ����Ϊ50g-25g=25g����25mL��
��������20g15%���������Һ����������粒����ˮ�������50g���ʵ���������Ϊ20%���������Һ��
50g���ʵ���������Ϊ20%���������Һ�к����������=50g×20%=10g
��20g15%���������Һ�к�������淋�����=20g×15%=3g
���Ի�Ӧ��������淋�����=10g-3g=7g
����Ҫˮ����Ϊ50g-20g-7g=23g����23mL����
�ʴ�Ϊ��
����һ��10gKNO3�����40gˮ��
��������25g40%��KNO3��Һ��25gˮ��
��������20g15%��KNO3��Һ��7gKNO3�����23gˮ��
b�����⣺ȡ12.5gʯ��ʯ��Ʒ��100g������ϡ���ᷴӦ��ֺ�������������Ϊ108.1g���ٲ���������̼������Ϊ���٣�����Ʒ��̼�����������Ϊ���٣��۷�Ӧ�����������ʵ����������Ƕ��٣�
�⣺�����ɵ�m��CO2��=12.5g+100g-108.1g=4.4g
����̼��Ƶ�����Ϊx����������������Ϊy�������Ȼ�������Ϊz
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73 111 44
x y z 4.4g
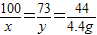
x=10g��y=7.3g��z=11.1g
��Ʒ��̼�����������=
 =80%
=80%�۷�Ӧ�����������ʵ���������=
 =51%
=51%�𣺢ٲ���������̼������Ϊ4.4g������Ʒ��̼�����������Ϊ80%���۷�Ӧ�����������ʵ�����������51%����ֻҪ���3��С�Ⲣ��Լ��ɣ�������������ⷨ���ɣ���
������������к�ǿ�Ŀ����ԣ����Բ�ȡ���ַ���������Һ�����ơ�����������ұ�д����ַ���ѧ���Ĵ����ԣ�

��ϰ��ϵ�д�
�����Ŀ
 33����������a��b��С�⣬��ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ƿ֣�
33����������a��b��С�⣬��ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ƿ֣�
 ��������a��b��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ʒ֣�
��������a��b��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ʒ֣�