��Ŀ����
��2013?̨�ݣ�ʵ���ҳ�������ķ������ռ�װ����ͼ��ʾ���ش��������⣺
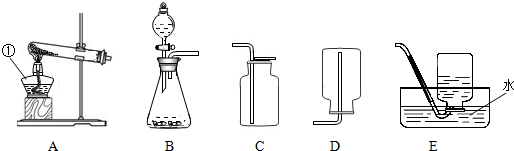
��1��ָ����Ţٵ���������
��2����ȡ������̼���壬Ӧѡ����ͼ�е�
��3��12.5��ʯ��ʯ��̼��Ƶ���������Ϊ80%�����ʲ����ᷴӦ���У�����������ϡ�����ַ�Ӧ�����Ƶö�����̼���ٿˣ�
��4�������������ռ��Ķ�����̼���ܻ��п������������һ����ʵ����֤��

��1��ָ����Ţٵ���������
�ƾ���
�ƾ���
����2����ȡ������̼���壬Ӧѡ����ͼ�е�
B��C
B��C
��ϣ�����ĸ��ţ�����3��12.5��ʯ��ʯ��̼��Ƶ���������Ϊ80%�����ʲ����ᷴӦ���У�����������ϡ�����ַ�Ӧ�����Ƶö�����̼���ٿˣ�
��4�������������ռ��Ķ�����̼���ܻ��п������������һ����ʵ����֤��
�����ж�����̼���Թܻ���ƿ����������������������Һ�У���۲쵽Һ��û�г��������������Ϊ�ռ��������к��п�������ͼҲ�ɣ�
�����ж�����̼���Թܻ���ƿ����������������������Һ�У���۲쵽Һ��û�г��������������Ϊ�ռ��������к��п�������ͼҲ�ɣ�
����ͼ������˵�����ɣ�����������1���ݳ��������ش�
��2���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã��ݶ�����̼���ܶȺ��ܽ���ѡ���ռ�װ�ã�
��3��ʯ��ʯ��������̼��Ƶ���������=̼��Ƶ���������̼��Ƶ��������̼��ƺ����ᷴӦ����ʽ�������ɶ�����̼��������
��4���ݶ�����̼���������Һ��Ӧ���ʵ�飮
��2���ݷ�Ӧ��״̬�ͷ�Ӧ����ѡ����װ�ã��ݶ�����̼���ܶȺ��ܽ���ѡ���ռ�װ�ã�
��3��ʯ��ʯ��������̼��Ƶ���������=̼��Ƶ���������̼��Ƶ��������̼��ƺ����ᷴӦ����ʽ�������ɶ�����̼��������
��4���ݶ�����̼���������Һ��Ӧ���ʵ�飮
����⣺��1����Ţٵ������Ǿƾ��ƣ�
��2��ʵ������ȡ������̼�ô���ʯ��ϡ���ᣬ������ȣ����ڹ�Һ�����ͣ���ѡ����װ��B��������̼���ܶȱȿ�������������ˮ�������������ſ������ռ���
��3��12.5��ʯ��ʯ��̼��Ƶ�������12.5g��80%=10g
�����ɶ�����̼������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
10g x
=
x=4.4g
�𣺿��Ƶö�����̼����4.4g��
��4�����ݶ�����̼���������Һ��Ӧ�����ʣ��ɽ����ж�����̼���Թܻ���ƿ����������������������Һ�У���۲쵽Һ��û�г������������ ��Ϊ�ռ��������к��п�����
�ʴ�Ϊ����1���ƾ��ƣ�
��2��B��C��
��3��4.4g��
��4�������ж�����̼���Թܻ���ƿ�����������ڼ���Һ�У���۲쵽Һ��û�г������������ ��Ϊ�ռ��������к��п�������ͼҲ�ɣ���
��2��ʵ������ȡ������̼�ô���ʯ��ϡ���ᣬ������ȣ����ڹ�Һ�����ͣ���ѡ����װ��B��������̼���ܶȱȿ�������������ˮ�������������ſ������ռ���
��3��12.5��ʯ��ʯ��̼��Ƶ�������12.5g��80%=10g
�����ɶ�����̼������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
10g x
| 100 |
| 10g |
| 44 |
| x |
x=4.4g
�𣺿��Ƶö�����̼����4.4g��
��4�����ݶ�����̼���������Һ��Ӧ�����ʣ��ɽ����ж�����̼���Թܻ���ƿ����������������������Һ�У���۲쵽Һ��û�г������������ ��Ϊ�ռ��������к��п�����
�ʴ�Ϊ����1���ƾ��ƣ�
��2��B��C��
��3��4.4g��
��4�������ж�����̼���Թܻ���ƿ�����������ڼ���Һ�У���۲쵽Һ��û�г������������ ��Ϊ�ռ��������к��п�������ͼҲ�ɣ���
���������⿼����ʵ������ȡ������̼��װ��ѡȡ��������̼�����ʼ���ؼ����֪ʶ���������֪ʶ������������������������⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��2013?���ݣ�ʵ��������һƿ��ǩ�������ɫҺ�壬��ͼ��ʾ����ƿ��ɫҺ����ʲô�أ�ʵ��Ա��ʦ���ߴ�ң���Һ��ֻ���ǹ���������Һ��ϡ���������ˮ�е�һ�֣�
��2013?���ݣ�ʵ��������һƿ��ǩ�������ɫҺ�壬��ͼ��ʾ����ƿ��ɫҺ����ʲô�أ�ʵ��Ա��ʦ���ߴ�ң���Һ��ֻ���ǹ���������Һ��ϡ���������ˮ�е�һ�֣�
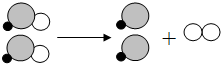 ��2013?̨�ݣ�����ˮ�г����д����ᣨHClO����������ȶ���������ѧ��Ӧ�����۱仯���̿�����ͼ��ʾ���÷�Ӧ�������ڣ�������
��2013?̨�ݣ�����ˮ�г����д����ᣨHClO����������ȶ���������ѧ��Ӧ�����۱仯���̿�����ͼ��ʾ���÷�Ӧ�������ڣ�������