��Ŀ����
��2009?��������ѧ����Ϊ����������˷ḻ�����ʻ���������Ϊ�������ɳ�����չ�����˾�Ĺ��ף�
��1��ú��ʯ�ͺ���Ȼ���ǵ�������Ҫ����Դ�����е�
��2���������������в���ȱ�ٵ�Ӫ�����ʣ����Тٰײ� ������ ������ ��ţ�⣬���и��������ʵ���
��3�����쿪����װ̼������ʱ���������������ӹ�ð����������˵��
��4��Ϊ�������˵����⣬���Բ�ȡ��һ�ַ�����
��1��ú��ʯ�ͺ���Ȼ���ǵ�������Ҫ����Դ�����е�
ʯ��
ʯ��
����Ϊ����ҵ��ѪҺ������2���������������в���ȱ�ٵ�Ӫ�����ʣ����Тٰײ� ������ ������ ��ţ�⣬���и��������ʵ���
��
��
������ţ�����3�����쿪����װ̼������ʱ���������������ӹ�ð����������˵��
ѹǿ
ѹǿ
���٣�������ܽ��Ҳ��֮��С����4��Ϊ�������˵����⣬���Բ�ȡ��һ�ַ�����
���ֲ˵��ྻ����
���ֲ˵��ྻ����
����������1�����ݻ�ʯ��Դ��Ӧ�÷�����
��2�����ݳ�����ʳƷ�к��е�Ӫ���ط�����
��3������������ܽ����ѹǿ�Ĺ�ϵ������
��4�����ݷ�ֹ������Ĵ�ʩ������
��2�����ݳ�����ʳƷ�к��е�Ӫ���ط�����
��3������������ܽ����ѹǿ�Ĺ�ϵ������
��4�����ݷ�ֹ������Ĵ�ʩ������
����⣺��1��ú��ʯ�ͺ���Ȼ���ǵ�������Ҫ����Դ���ڹ�ҵ�Ͼ��й㷺��Ӧ�ã�����ʯ�ͱ���Ϊ����ҵ��ѪҺ����
��2����ţ���к��зḻ�ĵ����ʣ��������������в���ȱ�ٵ�Ӫ�����ʣ�
��3�����쿪����װ̼������ʱ���������������ӹ�ð����������˵��ѹǿ���٣�������ܽ��Ҳ��֮��С��
��4��Ϊ�������˵����⣬���Բ�ȡ�ķ����϶࣬���磺���ֲ˵��ྻ����˵�����˺���Ϳ��ֲ���͵�
�ʴ�Ϊ����1��ʯ�ͣ���2���ܣ���3��ѹǿ�� ��4�����ֲ˵��ྻ����ȣ�
��2����ţ���к��зḻ�ĵ����ʣ��������������в���ȱ�ٵ�Ӫ�����ʣ�
��3�����쿪����װ̼������ʱ���������������ӹ�ð����������˵��ѹǿ���٣�������ܽ��Ҳ��֮��С��
��4��Ϊ�������˵����⣬���Բ�ȡ�ķ����϶࣬���磺���ֲ˵��ྻ����˵�����˺���Ϳ��ֲ���͵�
�ʴ�Ϊ����1��ʯ�ͣ���2���ܣ���3��ѹǿ�� ��4�����ֲ˵��ྻ����ȣ�
��������ѧ���������ϵ�dz����ܣ�ѧ��Ӧ�û�ѧ��֪ʶ�����������һЩ���⣮

��ϰ��ϵ�д�
 ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д� �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ
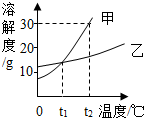 ��2009?��������ͼ�Ǽס������ֹ������ʵ��ܽ������ͼ������˵���в���ȷ���ǣ�������
��2009?��������ͼ�Ǽס������ֹ������ʵ��ܽ������ͼ������˵���в���ȷ���ǣ�������
