��Ŀ����
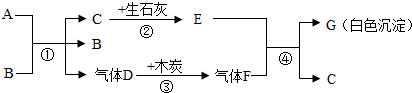
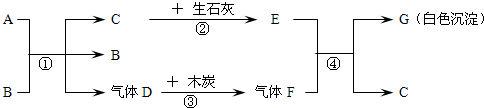
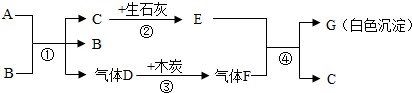
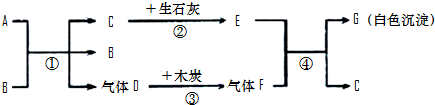
A��GΪ���ֳ��л�ѧ�г��������ʣ�AΪ��ɫҺ�壬BΪ��ɫ��ĩ��CΪ������ܼ���������ͼ��ʾ��ת����ϵ�����з�Ӧ���߱���Ӧ��Ӧ�����Ҿ���ȫ��Ӧ���ش��������⣺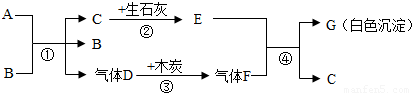
��1��A��G�Ļ�ѧʽ��A��G��
��2������B�ڷ�Ӧ���е����ÿ����ǣ�
��3������Ӣ�������������÷�Ӧ�ڡ��������̡����������ĽǶ������ǻ�ѧ��ת��Ϊ��
��4����д����Ӧ�٢ܵĻ�ѧ����ʽ���٣�
�ܣ�
���𰸡�����������Ľ���ͻ�ƿ�ΪCΪ������ܼ�����C����Ϊˮ��������ʯ�ҷ�Ӧ����E�������ƣ�A��B��ϵõ�ˮ������Dʱ����B����B�����Ǵ�����A����ʹ˫��ˮ��D����Ϊ������������ľ̿���ɵ�����FΪ������̼�������������Ʒ�Ӧ����G̼��Ƴ�����ˮ�������ͼ���ƶϺ�����
����⣺��1��CΪ������ܼ�����C����Ϊˮ��������ʯ�ҷ�Ӧ����E�������ƣ�A��B��ϵõ�ˮ������Dʱ����B����B�����Ǵ�����A����ʹ˫��ˮ��D����Ϊ������������ľ̿���ɵ�����FΪ������̼�������������Ʒ�Ӧ����G̼��Ƴ�����ˮ�����Ա����Ϊ��H2O2��CaCO3��
��2��A��B��ϵõ�ˮ������Dʱ����B����B�����Ǵ��������Ա����Ϊ��������
��3����ʯ����ˮ��Ӧ��������������ͬʱ�ų����������Ա����Ϊ�����ܣ�
��4�����������ڴ����������̵�������������ˮ�������������������������̼��Ӧ����̼��ƺ�ˮ�����Ա����Ϊ��2H2O2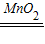 2H2O+O2����Ca��OH��2+CO2=CaCO3��+H2O��
2H2O+O2����Ca��OH��2+CO2=CaCO3��+H2O��
����������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ�ֱ�ӵó����ۣ�Ȼ������˳���������������м��ƣ���һ�����������ۣ�
����⣺��1��CΪ������ܼ�����C����Ϊˮ��������ʯ�ҷ�Ӧ����E�������ƣ�A��B��ϵõ�ˮ������Dʱ����B����B�����Ǵ�����A����ʹ˫��ˮ��D����Ϊ������������ľ̿���ɵ�����FΪ������̼�������������Ʒ�Ӧ����G̼��Ƴ�����ˮ�����Ա����Ϊ��H2O2��CaCO3��
��2��A��B��ϵõ�ˮ������Dʱ����B����B�����Ǵ��������Ա����Ϊ��������
��3����ʯ����ˮ��Ӧ��������������ͬʱ�ų����������Ա����Ϊ�����ܣ�
��4�����������ڴ����������̵�������������ˮ�������������������������̼��Ӧ����̼��ƺ�ˮ�����Ա����Ϊ��2H2O2
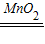 2H2O+O2����Ca��OH��2+CO2=CaCO3��+H2O��
2H2O+O2����Ca��OH��2+CO2=CaCO3��+H2O������������Ϊ��ͼʽ�����ƶ��⣬��ɴ�����Ŀ���ؼ���������ͻ�ƿڣ�ֱ�ӵó����ۣ�Ȼ������˳���������������м��ƣ���һ�����������ۣ�

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ