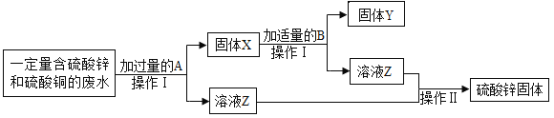
��Ŀ����
�������ڵ����ʲ������ֵ�һ���ǣ�������
A.NaOH��NaCl��Na2SO4������Һ ����̪�� B.Na2CO3��AgNO3��BaCl2������Һ ��ϡ���ᣩ
C.���ۡ�̼�ۡ�����ͭ��δ ��ϡ���ᣩ D.NaCl��NaOH��NH4NO3���ֹ��� ��ˮ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���ѧѧϰ�벻����ѧ�仯���仯ʵ�����ʼ���ת����
��1��Ca(OH)2����
����д��Ca(OH)2���ʵ�ԭ��______���û�ѧ����ʽ��ʾ����
��2�������о�Ca(OH)2���ʳ̶�
�������ʵ��ȷ��Ca(OH)2�ı��ʳ̶ȣ�д��������������______��
��3�������о�Ca(OH)2���ʳ̶�
������ϡ�����������300g��������Ϊ5%�����ᣬ��Ҫ��������Ϊ30%������______g��
���ƹ����У����ձ�������������Ͳ�⣬�����õ��IJ���������______������ȡ30%������ʱ���Ӷ����������Ƶ�������������______5%������ڡ�С�ڣ���
��ʵ��ⶨ����ȡ40.0g��Ʒ�����ձ��У���������ϡ�����ַ�Ӧ���ձ��������뷴Ӧʱ��Ĺ�ϵ�����ʾ��
��Ӧʱ��/min | 0 | t1 | t2 | t3 | t4 |
�ձ�������/g | 150.0 | 147.8 | 145.6 | 145.6 | 145.6 |
��������Ʒ��Ca(OH)2������������_____
KNO3��KCl�ڲ�ͬ�¶�ʱ���ܽ�������ʾ��
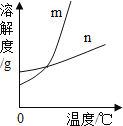
�¶�/�� | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 95.5 | 110 | 138 |
KC1 | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | 48.3 |
��1�����������ܽ����ȵ��¶ȷ�Χ��______�棬��ͼ�б�ʾKCl���ܽ��������______���m����n������
��2��40��ʱ����40gKCl���뵽50gˮ�У���������Һ����������Ϊ______��