��Ŀ����
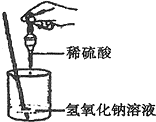 ��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ��
��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ��С��˵������ֻ�������ƣ�
�ϴ�˵�����ʳ��������ƻ���ϡ����������룬���û�������һ�ֿ��ܣ����������ƻ���
Ϊ��֤����ҵIJ��룬С�����ձ���ȡ��������Ӧ�����Һ��һ֧�Թ��У������Թ��еμӼ�����ɫ��̪��Һ�����۲쵽��̪��Һ����ɫ������С���ʹϴ�˵��IJ����Dz���ȷ��
��1������Ϊ�����ó��Ľ����Ƿ���ȷ
��2��Ϊ�˽�һ��̽��С���ʹϴϵIJ����ĸ���ȷ����Ҫ���������һ��ʵ�飬��д�±���
| ʵ�鷽�� | ���ܹ۲쵽������ | ���� |
��ʵ���У������μӷ�̪��Һ������ʯ����Һ��ԭ��
��ʵ������У�Ҫ�ò��������Ͻ��裬��������Ŀ����
��������ʵ����������ⷢ�������ݳ��֣�����Ϊ���ܵ�ԭ����
��4��Ϊ�˼����˽��������Ʊ��ʵ��������ȡ4g�������ƹ������ձ��У��μ����ʵ���������Ϊ10%��ϡ���ᵽ���ٲ�������Ϊֹ�����ռ���1.1g������̼���������Ʒ���������Ƶ�����������
�����������������ƺ����ᶼ�й����Ŀ��ܻ�������ǡ����ȫ��Ӧ���з������룻
��1�����ݼ��ʹ��̪��ɫ���з�����
��2�����������̼���η�Ӧ���ɶ�����̼����������ʵ�飻
��3���ٸ���ʯ��ı�ɫ�Ǵ���ɫ����ɫ�仯�������ڹ۲���з������ڸ��ݽ������ʹ��Һ��ֻ�Ϸ�Ӧ���з������۸����������Ʊ��ʵ�ԭ���������ʵķ���������
��4������̼���ƺ����ᷴӦ�Ļ�ѧ����ʽ���������ɶ�����̼����������������̼���Ƶ��������ٸ���̼�������������������������Ƶ������������ݴ��ȹ�ʽ�����������������Ƶ�����������
��1�����ݼ��ʹ��̪��ɫ���з�����
��2�����������̼���η�Ӧ���ɶ�����̼����������ʵ�飻
��3���ٸ���ʯ��ı�ɫ�Ǵ���ɫ����ɫ�仯�������ڹ۲���з������ڸ��ݽ������ʹ��Һ��ֻ�Ϸ�Ӧ���з������۸����������Ʊ��ʵ�ԭ���������ʵķ���������
��4������̼���ƺ����ᷴӦ�Ļ�ѧ����ʽ���������ɶ�����̼����������������̼���Ƶ��������ٸ���̼�������������������������Ƶ������������ݴ��ȹ�ʽ�����������������Ƶ�����������
����⣺�������ƺ������ڷ����кͷ�Ӧʱ���������ƺ����ᶼ�й����Ŀ��ܻ�������ǡ����ȫ��Ӧ���ʴ�Ϊ���������ƣ�
��1����������������ڣ������̪���ͻ��ɺ�ɫ�����Ǹ���Һ�����̪����ɫ��˵����û���������ƣ��ʴ�Ϊ����ȷ����Һ����ɫ˵��û���������ƣ�
��2���������̼���Ʒ�Ӧ���ɶ�����̼���壬���Լ���̼���Ƽ�������Ĵ��ڣ��ʴ�Ϊ��ȡ��Һ���Թ��У��μ�̼������Һ����������ݲ�����˵����ϡ���ᣬ�ϴ���ȷ���������û���ݣ�˵��û��ϡ���ᣬС����ȷ����
��3���ټ���ʯ����ָʾ����ʯ���������ɫ����������Һ����ɫ��ǡ���к�ʱ��ɫ����ɫ�����ɫ�������۲죬�ʴ�Ϊ����Һ����ɫ���ɵ���ɫ����ɫ�������ԣ������÷�̪��Һ��
�ڽ������ʹ��Һ��ֽӴ����ӿ췴Ӧ���ʴ�Ϊ��ʹ���������������ַ�Ӧ�����������Ʊ�¶�ڿ������������տ����еĶ�����̼����̼���ƶ����ʣ����ʺ����ɵ�̼���ƻ�ͼ�������ᷴӦ���������ơ�ˮ�Ͷ�����̼
�ʴ�Ϊ��2NaOH+CO2=Na2CO3+H2O��Na2CO3+H2SO4=Na2SO4+H2O+C02����
�ʴ�Ϊ���������ƣ�
��1����ȷ����Һ����ɫ˵��û���������ƣ�
��2��ȡ��Һ���Թ��У��μ�̼������Һ����������ݲ�����˵����ϡ���ᣬ�ϴ���ȷ�������û���ݣ�˵��û��ϡ���ᣬС����ȷ����
��3������Һ����ɫ���ȵ���ɫ����ɫ�������ԣ������÷�̪��Һ��
��ʹ���������������ַ�Ӧ��
��2NaOH+CO2=Na2CO3+H2O��Na2CO3+H2SO4=Na2SO4+H2O+C02����
��4���⣺��̼���Ƶ�����ΪX
Na2CO3+H2SO4=Na2SO4+H2O+C02��
106 44
X 1.1g
=
X=2.65g
��100%=33.75%
���������Ƶ���������Ϊ33.75%
��1����������������ڣ������̪���ͻ��ɺ�ɫ�����Ǹ���Һ�����̪����ɫ��˵����û���������ƣ��ʴ�Ϊ����ȷ����Һ����ɫ˵��û���������ƣ�
��2���������̼���Ʒ�Ӧ���ɶ�����̼���壬���Լ���̼���Ƽ�������Ĵ��ڣ��ʴ�Ϊ��ȡ��Һ���Թ��У��μ�̼������Һ����������ݲ�����˵����ϡ���ᣬ�ϴ���ȷ���������û���ݣ�˵��û��ϡ���ᣬС����ȷ����
��3���ټ���ʯ����ָʾ����ʯ���������ɫ����������Һ����ɫ��ǡ���к�ʱ��ɫ����ɫ�����ɫ�������۲죬�ʴ�Ϊ����Һ����ɫ���ɵ���ɫ����ɫ�������ԣ������÷�̪��Һ��
�ڽ������ʹ��Һ��ֽӴ����ӿ췴Ӧ���ʴ�Ϊ��ʹ���������������ַ�Ӧ�����������Ʊ�¶�ڿ������������տ����еĶ�����̼����̼���ƶ����ʣ����ʺ����ɵ�̼���ƻ�ͼ�������ᷴӦ���������ơ�ˮ�Ͷ�����̼
�ʴ�Ϊ��2NaOH+CO2=Na2CO3+H2O��Na2CO3+H2SO4=Na2SO4+H2O+C02����
�ʴ�Ϊ���������ƣ�
��1����ȷ����Һ����ɫ˵��û���������ƣ�
��2��ȡ��Һ���Թ��У��μ�̼������Һ����������ݲ�����˵����ϡ���ᣬ�ϴ���ȷ�������û���ݣ�˵��û��ϡ���ᣬС����ȷ����
��3������Һ����ɫ���ȵ���ɫ����ɫ�������ԣ������÷�̪��Һ��
��ʹ���������������ַ�Ӧ��
��2NaOH+CO2=Na2CO3+H2O��Na2CO3+H2SO4=Na2SO4+H2O+C02����
��4���⣺��̼���Ƶ�����ΪX
Na2CO3+H2SO4=Na2SO4+H2O+C02��
106 44
X 1.1g
| 106 |
| x |
| 44 |
| 1.1g |
X=2.65g
| 4g-2.65g |
| 4g |
���������Ƶ���������Ϊ33.75%
���������������кͷ�Ӧ��̽�����ڽ������ʱ������Ӧ��ѧ����֪ʶ��������еIJ��룬Ȼ���ٸ����ᡢ��ε����ʺ�ָʾ���ı仯������

��ϰ��ϵ�д�
�����Ŀ
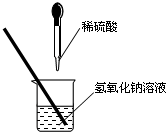 ��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ��
��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ�� ��2013?������һģ����ͼ��ʾ��ij��ȤС���ڽ�������кͷ�Ӧ��ʵ��̽��ʱ�����ձ�������������Һ�еμ�ϡ����һ����������ǵμ�ָʾ������ͬѧ���ձ���ȡ������Ӧ�����Һ��һ֧�Թ��У������Թ��еμӼ�����ɫ��̪��Һ�����۲쵽��̪��Һ����ɫ��
��2013?������һģ����ͼ��ʾ��ij��ȤС���ڽ�������кͷ�Ӧ��ʵ��̽��ʱ�����ձ�������������Һ�еμ�ϡ����һ����������ǵμ�ָʾ������ͬѧ���ձ���ȡ������Ӧ�����Һ��һ֧�Թ��У������Թ��еμӼ�����ɫ��̪��Һ�����۲쵽��̪��Һ����ɫ��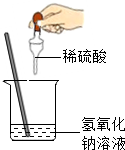 ��2013?����ģ�⣩��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ��
��2013?����ģ�⣩��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ��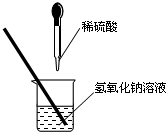 ��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ��
��ͼ��ʾ��ij��ȤС��ͬѧ�ڽ�������кͷ�Ӧ��ʵ��ʱ�����ձ��е�����������Һ�μ�ϡ���ᣬһ����������������˵μӷ�ָ̪ʾ���������ȷ��ϡ���������������Ƿ�ǡ����ȫ��Ӧ��