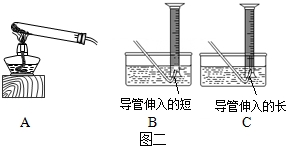
��Ŀ����
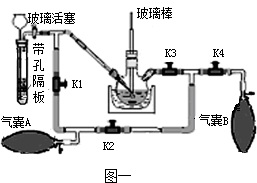
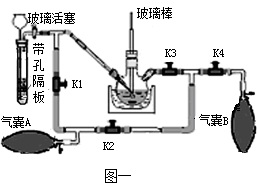
������ؿ���ɱ��ϸ�����Ǽ�ͥ�ر��ij�������ҩ����֪��K2MnO4��ҺΪ��ɫ��Һ��KMnO4����ȡ��
ʵ������ȡKMnO4�ķ����Ͳ����Ǣٸ�������MnO2��KOH�Ȼ��K2MnO4�����������������ˮ�ܽ�K2MnO4�ۼ���K2MnO4��Һ������ͨ��CO2����Ӧ����KMnO4��K2CO3��MnO2������Һ�����ˣ��������º�ɣ������Ƶø�����ؾ��壮
��1�����������������ˮ�ܽ��Ŀ����______��������л�ȡKMnO4�Ļ�ѧ����ʽ��______ 2KMnO4+2K2CO3+MnO2
���𰸡���������1���ڸ����¶������ܽ����ʼӿ�ĵ�������������������ˮ�ܽ�K2MnO4��ԭ��֪����Ӧ����������Լ���Ӧ������Ȼ����ƽ��ѧ����ʽ�ͺ��ˣ��ܸ�����������ֽ⣻
��2��ʵ�����ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ����ȡ������̼��
��3������������Ϻ�ɫ�Ĺ��壻
��4����Ϊʵ���Լ�˳ɱ��ͻ����ľ��ȿ������⣻
��5����ˮ���ռ����ɵ�����ʱ�����ܲ�������̫����Ϊ�˷�ֹ������ض������ܣ�ʵ��ʱͨ�����Թܿ���һ������
��6��Ϊ�˱�������IJ���Ҫ�ݳ��������ʵ�����ݵIJ���ȷ�������ڲ�����1�������ǾͿ�ʼ�ռ����壻������������������͵ĵ�����δ��ȴ�Ͷ�ȡ��Ͳ�������������ʹ�òⶨ���������ƫ��
��7�����ݻ�ѧ����ʽ��ʾ�����Ĺ�ϵ���м��㣻
��8�����ݻ�ѧ����ʽ��ʾ�����Ĺ�ϵ���м��㣻
��9��������������������͵ĵ�����δ��ȴ�Ͷ�ȡ��Ͳ�������������ʹ�òⶨ���������ƫ��
����⣺��1���ڸ����¶������ܽ����ʼӿ�ĵ�������������������ˮ�ܽ�K2MnO4��ԭ��
�۴���Ŀ����������K2MnO4��Һ������ͨ��CO2����Ӧ����KMnO4��K2CO3��MnO2����֪����Ӧ����K2MnO4��CO2����������KMnO4��K2CO3��MnO2��Ӧ�����Ǽ��ȣ�Ȼ����д����ƽ��ѧ����ʽ�ͺ��ˣ�
�����ڸ�����������ֽ⣬���Ը�����ؾ���ֻ�ܵ��º�ɣ�
�ʴ�Ϊ���ӿ��ܽ����ʣ�3K2MnO4+2CO2 2KMnO4+2K2CO3+MnO2�������ֽ⣻
2KMnO4+2K2CO3+MnO2�������ֽ⣻
��2��ʵ�����ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ����ȡ������̼���ʴ�Ϊ������ʯ��ʯ��ʯ��ϡ���
��3������������Ϻ�ɫ�Ĺ��壬�ʴ�Ϊ��K4��K3���Ϻ죻
��4����������ʹ������̼�ﵽ�����������ʣ��Ӷ�Ϊʵ���Լ�˳ɱ���������һ�ֻ������������ʴ�Ϊ����Լ�ɱ���CO2�������ʴ������������𰸣�
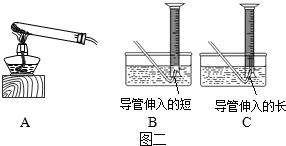
��5����ˮ���ռ����ɵ�����ʱ��Ϊ����ˮ�����ų��������Ե��ܲ�������̫����Ϊ�˷�ֹ������ض������ܣ�ʵ��ʱͨ�����Թܿ���һ�������ʴ�Ϊ��B����ֹ������ض������ܣ�
��6��Ϊ�˱�������IJ���Ҫ�ݳ��������ʵ�����ݵIJ���ȷ�������ڲ�����1�������ǾͿ�ʼ�ռ����壻������������������͵ĵ�������ȡ�������ǰ��Ҫ��Ϩ��ƾ��ƣ�ʹ�¶Ƚ��͵����£��������������¶�̫���������õ��������ƫ����Ϊ����һֱ�dz��µĻ�����ô�൱�������ɵ������������ż������ģ�����������������û�н�����Ͳ�����������ȴ����Ͳ�����ֳ����ˣ����ʴ�Ϊ��������1�����ݣ�Ϩ��
��7������O2���������Ϊ��1.420g/L×0.1L=0.1420g���ݻ�ѧ����ʽ���㣬�������ص�����Ϊx��
2KMnO4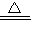 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��
316 32
x 0.1420g
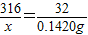
��ã�x=1.40g
�ʴ�Ϊ��1.40g
��8���⣺����Ʒ�и�����صĺ���Ϊx��O2������Ϊ56.3×1.420=0.08g
2KMnO4 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��
316 32
X 0.08g
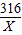 =
=
X=0.79g
��9��������������������͵ĵ�����δ��ȴ�Ͷ�ȡ��Ͳ�������������ʹ�òⶨ���������ƫ���ݻ�ѧ����ʽ��ʾ��������ϵ���Ϳ���֪���ⶨ��KMnO4��Ʒ����ƫ�ʴ�Ϊ��δ��ȴ�Ͷ�ȡ��Ͳ���������������������Ҳ�ɣ���
�������������漰��֪ʶ��࣬���Խ���������ѣ�ͬѧ���ڿ���ʱ���µ����������ˣ�
��2��ʵ�����ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ����ȡ������̼��
��3������������Ϻ�ɫ�Ĺ��壻
��4����Ϊʵ���Լ�˳ɱ��ͻ����ľ��ȿ������⣻
��5����ˮ���ռ����ɵ�����ʱ�����ܲ�������̫����Ϊ�˷�ֹ������ض������ܣ�ʵ��ʱͨ�����Թܿ���һ������
��6��Ϊ�˱�������IJ���Ҫ�ݳ��������ʵ�����ݵIJ���ȷ�������ڲ�����1�������ǾͿ�ʼ�ռ����壻������������������͵ĵ�����δ��ȴ�Ͷ�ȡ��Ͳ�������������ʹ�òⶨ���������ƫ��
��7�����ݻ�ѧ����ʽ��ʾ�����Ĺ�ϵ���м��㣻
��8�����ݻ�ѧ����ʽ��ʾ�����Ĺ�ϵ���м��㣻
��9��������������������͵ĵ�����δ��ȴ�Ͷ�ȡ��Ͳ�������������ʹ�òⶨ���������ƫ��
����⣺��1���ڸ����¶������ܽ����ʼӿ�ĵ�������������������ˮ�ܽ�K2MnO4��ԭ��
�۴���Ŀ����������K2MnO4��Һ������ͨ��CO2����Ӧ����KMnO4��K2CO3��MnO2����֪����Ӧ����K2MnO4��CO2����������KMnO4��K2CO3��MnO2��Ӧ�����Ǽ��ȣ�Ȼ����д����ƽ��ѧ����ʽ�ͺ��ˣ�
�����ڸ�����������ֽ⣬���Ը�����ؾ���ֻ�ܵ��º�ɣ�
�ʴ�Ϊ���ӿ��ܽ����ʣ�3K2MnO4+2CO2
 2KMnO4+2K2CO3+MnO2�������ֽ⣻
2KMnO4+2K2CO3+MnO2�������ֽ⣻��2��ʵ�����ô���ʯ��ʯ��ʯ��ϡ���ᷴӦ����ȡ������̼���ʴ�Ϊ������ʯ��ʯ��ʯ��ϡ���
��3������������Ϻ�ɫ�Ĺ��壬�ʴ�Ϊ��K4��K3���Ϻ죻
��4����������ʹ������̼�ﵽ�����������ʣ��Ӷ�Ϊʵ���Լ�˳ɱ���������һ�ֻ������������ʴ�Ϊ����Լ�ɱ���CO2�������ʴ������������𰸣�
��5����ˮ���ռ����ɵ�����ʱ��Ϊ����ˮ�����ų��������Ե��ܲ�������̫����Ϊ�˷�ֹ������ض������ܣ�ʵ��ʱͨ�����Թܿ���һ�������ʴ�Ϊ��B����ֹ������ض������ܣ�
��6��Ϊ�˱�������IJ���Ҫ�ݳ��������ʵ�����ݵIJ���ȷ�������ڲ�����1�������ǾͿ�ʼ�ռ����壻������������������͵ĵ�������ȡ�������ǰ��Ҫ��Ϩ��ƾ��ƣ�ʹ�¶Ƚ��͵����£��������������¶�̫���������õ��������ƫ����Ϊ����һֱ�dz��µĻ�����ô�൱�������ɵ������������ż������ģ�����������������û�н�����Ͳ�����������ȴ����Ͳ�����ֳ����ˣ����ʴ�Ϊ��������1�����ݣ�Ϩ��
��7������O2���������Ϊ��1.420g/L×0.1L=0.1420g���ݻ�ѧ����ʽ���㣬�������ص�����Ϊx��
2KMnO4
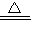 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��316 32
x 0.1420g
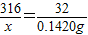
��ã�x=1.40g
�ʴ�Ϊ��1.40g
��8���⣺����Ʒ�и�����صĺ���Ϊx��O2������Ϊ56.3×1.420=0.08g
2KMnO4
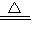 K2MnO4+MnO2+O2��
K2MnO4+MnO2+O2��316 32
X 0.08g
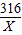 =
=
X=0.79g
��9��������������������͵ĵ�����δ��ȴ�Ͷ�ȡ��Ͳ�������������ʹ�òⶨ���������ƫ���ݻ�ѧ����ʽ��ʾ��������ϵ���Ϳ���֪���ⶨ��KMnO4��Ʒ����ƫ�ʴ�Ϊ��δ��ȴ�Ͷ�ȡ��Ͳ���������������������Ҳ�ɣ���
�������������漰��֪ʶ��࣬���Խ���������ѣ�ͬѧ���ڿ���ʱ���µ����������ˣ�

��ϰ��ϵ�д�
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
�����Ŀ