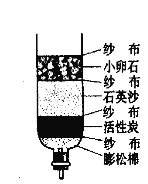
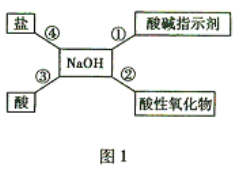
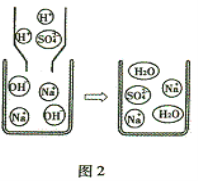
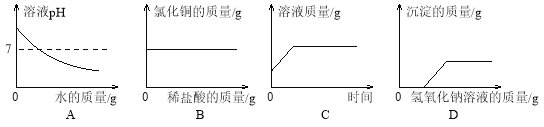
��Ŀ����
����Ŀ����7�֣�ij�о���ѧϰС�鵽��������һ�����棬��װ���ϱ�����Ҫ�ɷ���̼���ƣ��������������Ȼ��ơ�����ȤС��Ϊ�о���ɷ֣���ȡ��Ʒ25.0g���������Ƴ���Һ������������μ���������������������Ϊ14.6%��ϡ���ᣬ��Ӧ���ɶ�����̼���������������ϡ����������ϵ��ͼ���Իش��������⣺
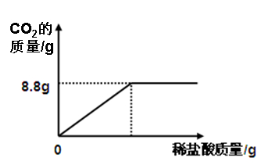
��1����Ʒ��ַ�Ӧ������CO2������Ϊ g
��2��ԭ������Na2CO3�����������Ƕ��٣�����д��������̣�
��3����Ʒ��̼����������ǡ����ȫ��Ӧʱ�����ĵ�ϡ�����������_____________g
��������˼���о���ѧϰС��ļ�ͬѧ��Ϊ��Ҫ���̼���Ƶ�������������ʹ���������������ͬ���������ʣ���________�� ______����һ�־������ʵĻ�ѧʽ����������������𣩵���Һ����Ʒ��Ӧ���ⶨ������ʵ������������йؼ��㼴�ɡ�
���𰸡���1�� 8.8 g��2��84.8%��3��100g��������˼��CaCl2 �� ��
��������
����������裺��Ʒ��̼���Ƶ�����Ϊx ����Ҫ���������ΪY
Na2CO3 + 2HCl == 2NaCl + CO2 �� + H2O
106 73 44
x Y 8.8g
�б���ʽ��:106��X=44:8.8�� ��ã�X=21.2�ˣ�ͬ�����Y=14.6��
��ô�����Ʒ��̼���Ƶ���������Ϊ21.2g/25.0g �� 100% == 84.8%
��Ʒ��̼����������ǡ����ȫ��Ӧʱ�����ĵ�ϡ�����������=14.6��14.6%=100��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�