��Ŀ����
ʵ����������Һʱ�������漰���²������ٳ����ڹ��ˢۼ���������ᾧ ���ܽ⣮�밴Ҫ��ش��������⣺��1����ͬѧ�ô������Ȼ��ƹ��������ˮ����100��������������Ϊ15%���Ȼ�����Һ���漰���IJ�������ȷ˳���� ______������ţ���
��2������������Һ��Ҫˮ������� ______mL��ˮ���ܶ���l g/cm3�������õ��IJ����������ձ�����Ͳ�� ______ʵ������10mL��50mL��100mL���ֲ�ͬ������Ͳ������������Һʱ���ѡ�� ______mL����Ͳ��
��3����ͬѧ�ú����ʵ�ʳ�Σ����ʲ�����ˮ��������ˮ�����Ȼ�����Һ������ǰӦ�����ᴿ����ô���ᴿʳ���漰���IJ�������ȷ˳���� ______������ţ���
���𰸡���������1��������Һʱ����Ҫͨ��������ȷ�������ʺ��ܼ�������Ȼ����г���������ܽ⣻
��2��������������������ʽ���Լ��������ˮ�������Ȼ�����ˮ�����ѡ��������̵���Ͳ��
��3�������к��е��Dz����Թ������ʣ�����ͨ�����˵ķ�����ȥ�������ᴿ��һ�㲽�����ܽ⡢���ˡ�������
����⣺��1���������ʼ�ˮ�ܽ�����Ʋ���Ϊ���㡢�������ܽ⣬���Ա����Ϊ���ۢ٢ݣ�
��2������100��������������Ϊ15%���Ȼ�����Һ�������Ȼ��Ƶ�����Ϊ100g×15%=15g������ˮ�����Ϊ85mL��������Ҫѡ��100mL����Ͳ�����õIJ���������Ҫ���ձ�����Ͳ�������������Ա����Ϊ��85����������100��
��3�������к��е��Dz����Թ������ʣ�����ͨ�����˵ķ�����ȥ�������ᴿ��һ�㲽�����ܽ⡢���ˡ����������Ա����Ϊ���ݢڢܣ�
������������Һ�����������������ʼ�ˮ�ܽ⣬���Ʋ�����㡢�������ܽ⣻�����ᴿ��һ�㲽��Ϊ�ܽ⡢���ˡ�������
��2��������������������ʽ���Լ��������ˮ�������Ȼ�����ˮ�����ѡ��������̵���Ͳ��
��3�������к��е��Dz����Թ������ʣ�����ͨ�����˵ķ�����ȥ�������ᴿ��һ�㲽�����ܽ⡢���ˡ�������
����⣺��1���������ʼ�ˮ�ܽ�����Ʋ���Ϊ���㡢�������ܽ⣬���Ա����Ϊ���ۢ٢ݣ�
��2������100��������������Ϊ15%���Ȼ�����Һ�������Ȼ��Ƶ�����Ϊ100g×15%=15g������ˮ�����Ϊ85mL��������Ҫѡ��100mL����Ͳ�����õIJ���������Ҫ���ձ�����Ͳ�������������Ա����Ϊ��85����������100��
��3�������к��е��Dz����Թ������ʣ�����ͨ�����˵ķ�����ȥ�������ᴿ��һ�㲽�����ܽ⡢���ˡ����������Ա����Ϊ���ݢڢܣ�
������������Һ�����������������ʼ�ˮ�ܽ⣬���Ʋ�����㡢�������ܽ⣻�����ᴿ��һ�㲽��Ϊ�ܽ⡢���ˡ�������

��ϰ��ϵ�д�
�����Ŀ


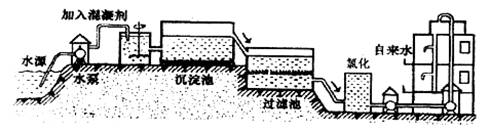
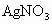 ��Һʱ����ʹ������ˮ����ԭ����(�û�ѧ����ʽ��ʾ)________���������
��Һʱ����ʹ������ˮ����ԭ����(�û�ѧ����ʽ��ʾ)________��������� ��һ�������ˮ�������������������Ԫ�صĻ��ϼ���________��
��һ�������ˮ�������������������Ԫ�صĻ��ϼ���________��
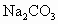 ��
��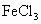 ��NaOH��HCl�е�һ�֣�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ�ʺ��ɫ��������ˮ�ɻ���壻�����������ݣ���ˮ���壮��ش�
��NaOH��HCl�е�һ�֣�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ�ʺ��ɫ��������ˮ�ɻ���壻�����������ݣ���ˮ���壮��ش�