��Ŀ����
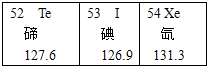
��Ԫ����Ԫ�����ڱ��е�λ����ͼ��ʾ�������Ե�—l31(��131���Ǹõ�ԭ���е���������������֮��)�Ǻ˷�Ӧ�IJ������˥��ʱ�����˷��䡣�˷��õ�Ƭ��KI���������յ�ʹ��״���ڵĵⱥ�ͣ��Ӷ���ֹ�����Ե�����롣
(1)��Ԫ�ص�ԭ������Ϊ ��
(2)��—l31ԭ�����Ԫ�ص�����ԭ�������ͬ��Ŀ�������������—131ԭ���е�������Ϊ ��
(3)��Ƭ�е���Ч�ɷ��� Ԫ�ء�[��Դ:ѧ����]
| 52 Te �� 127.6 | 53 I �� 126.9 | 54 Xe � 131.3 |
(1����O2��H2O��CO��NaOH��NaHCO3
��2��2NaH CO3![]() Na2CO3 + H2O + CO2�� 2H2O
Na2CO3 + H2O + CO2�� 2H2O ![]()
![]() 2H2��+ O2 ��
2H2��+ O2 ��
![]() ��3��I����⣩
��3��I����⣩

��ϰ��ϵ�д�
 ̽���빮�̺��Ͽ�ѧ����������ϵ�д�
̽���빮�̺��Ͽ�ѧ����������ϵ�д�
�����Ŀ
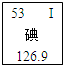 7��2011��3���ձ�����������˺�й©�¹ʣ���й©�ͷų��˷����Ե�-131������Ӵ���-131�����������״�ټ�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ��ʾ������˵����ȷ���ǣ�������
7��2011��3���ձ�����������˺�й©�¹ʣ���й©�ͷų��˷����Ե�-131������Ӵ���-131�����������״�ټ�������Ԫ����Ԫ�����ڱ��е���Ϣ��ͼ��ʾ������˵����ȷ���ǣ�������