��Ŀ����
�۲������ȼ�գ��ó����н��ۣ���ش�
��1����Ҫȼ�վ��ܹ۲쵽����������ĸ��
��2����Ҫȼ�ղ��ܹ۲쵽����������ĸ��
a���Զ�����Լ1cm���ڵ��������µġ������������ԣ�
b��ԭ���ǰ�����������ζ��ɫ���壻
c�����ˣ����ĵײ���Χ����ɫҺ�壬����״���ڣ�
d��һ��ɫ���Ĺᴩ���ᣬ���ӳ�������1cm��
e����״Բ���Σ�����ΪԲ�μ����
f��������Χ�������϶˳������Ļ�ɫ��
g����Ϩ���ܿ���������Χ�а�����������������ʧ��
h������������ָ�γ��ۼ���
i�����紵������һ�ߣ���һ�����ζ��˵������۳�Һ�岢���������£�
j�����ܿ�������Ӱ�죬��������������������̣�
����֮�⣬ȼ��ʱ�㻹�۲쵽ʲô��������д������
��3�������Ϩ��ʱ�ܻ���һ�ư���ð�������ijɷ���ʲô�أ�������������¼��裺
A��������ȼ��ʱ���ɵĶ�����̼
B��������ȼ��ʱ���ɵ�ˮ����
C��������ʯ���������ɵ�ʯ������
ʵ����֤��
�ٴ�������������һ��մ�г���ʯ��ˮ���ձ���ס���̣���Ŀ����Ϊ����֤����
�ڴ�������������һ��ɶ������IJ���Ƭ���ڰ�����������Ƭ��û�г���ˮ����˵�����̲���
�۴�������������ȼ�ŵ�ľ��ȥ����̣�ע�ⲻҪ�Ӵ���о���������������±���ȼ��˵�����̾��п�ȼ�ԣ���Ϊ����
��4��ȡ10g������������������ȼ�գ�����8.8g�� ����̼ ��7.2gˮ��ͨ�������ж������Ƿ�����Ԫ�أ�
��1����Ҫȼ�վ��ܹ۲쵽����������ĸ��
abdeh
abdeh
����2����Ҫȼ�ղ��ܹ۲쵽����������ĸ��
cfgij
cfgij
a���Զ�����Լ1cm���ڵ��������µġ������������ԣ�
b��ԭ���ǰ�����������ζ��ɫ���壻
c�����ˣ����ĵײ���Χ����ɫҺ�壬����״���ڣ�
d��һ��ɫ���Ĺᴩ���ᣬ���ӳ�������1cm��
e����״Բ���Σ�����ΪԲ�μ����
f��������Χ�������϶˳������Ļ�ɫ��
g����Ϩ���ܿ���������Χ�а�����������������ʧ��
h������������ָ�γ��ۼ���
i�����紵������һ�ߣ���һ�����ζ��˵������۳�Һ�岢���������£�
j�����ܿ�������Ӱ�죬��������������������̣�
����֮�⣬ȼ��ʱ�㻹�۲쵽ʲô��������д������
��3�������Ϩ��ʱ�ܻ���һ�ư���ð�������ijɷ���ʲô�أ�������������¼��裺
A��������ȼ��ʱ���ɵĶ�����̼
B��������ȼ��ʱ���ɵ�ˮ����
C��������ʯ���������ɵ�ʯ������
ʵ����֤��
�ٴ�������������һ��մ�г���ʯ��ˮ���ձ���ס���̣���Ŀ����Ϊ����֤����
A
A
������ţ����ڴ�������������һ��ɶ������IJ���Ƭ���ڰ�����������Ƭ��û�г���ˮ����˵�����̲���
B
B
���۴�������������ȼ�ŵ�ľ��ȥ����̣�ע�ⲻҪ�Ӵ���о���������������±���ȼ��˵�����̾��п�ȼ�ԣ���Ϊ����
C
C
������ţ��ṩ��֤�ݣ�ͬʱ�ų��˼���AB
AB
������ţ�����Ϊ������̼��ˮ����������ȼ��
������̼��ˮ����������ȼ��
����4��ȡ10g������������������ȼ�գ�����8.8g�� ����̼ ��7.2gˮ��ͨ�������ж������Ƿ�����Ԫ�أ�
��������1������Ҫȼ�վ��ܹ۲쵽������һ��ָ����������������ʣ�
��2��ȼ��ʱ������һ���ǻ�ѧ�仯ʱ���ֵ������ֻ���ʱ�����ڱ���
��3��ʵ����֤���ٸ��ݶ�����̼��ʹ����ʯ��ˮ����ǵ������ж�����֤���ּ��輴�ɣ�
�ڴ�������������һ��ɶ���IJ���Ƭ���ڰ����ϣ�����Ƭ��û��ˮ������Ϊ֤���Ƿ���ˮ������
�۴�����������ȼ�ŵ�ľ��ȥ����̣���Ҫ�Ӵ���о���������������±���ȼ��˵�����̾��п�ȼ�ԣ���Ϊ����C�ṩ���ݣ�����Ϊˮ�����Ͷ�����̼������ȼ�գ�����ͬʱ���ų�����AB�����Ծݴ�����ɸ���Ľ��
��4���������ɶ�����̼�������Ͷ�����̼��̼Ԫ�ص�������������������л����к���C��������ͬ������������ˮ��������H2O����Ԫ�ص�������������������л����к���H���������ٸ��������غ㶨�ɣ���Ӧǰ���л��������Ƿ�������ɵ�C��H���������ݴ˼����жϸ��л������Ƿ�����Ԫ�أ�
��2��ȼ��ʱ������һ���ǻ�ѧ�仯ʱ���ֵ������ֻ���ʱ�����ڱ���
��3��ʵ����֤���ٸ��ݶ�����̼��ʹ����ʯ��ˮ����ǵ������ж�����֤���ּ��輴�ɣ�
�ڴ�������������һ��ɶ���IJ���Ƭ���ڰ����ϣ�����Ƭ��û��ˮ������Ϊ֤���Ƿ���ˮ������
�۴�����������ȼ�ŵ�ľ��ȥ����̣���Ҫ�Ӵ���о���������������±���ȼ��˵�����̾��п�ȼ�ԣ���Ϊ����C�ṩ���ݣ�����Ϊˮ�����Ͷ�����̼������ȼ�գ�����ͬʱ���ų�����AB�����Ծݴ�����ɸ���Ľ��
��4���������ɶ�����̼�������Ͷ�����̼��̼Ԫ�ص�������������������л����к���C��������ͬ������������ˮ��������H2O����Ԫ�ص�������������������л����к���H���������ٸ��������غ㶨�ɣ���Ӧǰ���л��������Ƿ�������ɵ�C��H���������ݴ˼����жϸ��л������Ƿ�����Ԫ�أ�
����⣺��1���ص�Ӧ�ù�ע�����һЩ�������ԣ���ѡ���е�abdeh�������ʵ��������ԣ�
��2���ص�Ӧ���ҵ�����ȼ�ճ��ֻ����һЩ��ѧ���ʣ�cfgij��������ȼ�չ����вű��ֳ��������ʣ�Ȼ����ʵ���Ҿƾ��ƻ����һЩ����дһЩ������û�е������磺�����Ϊ3�㣻��������������
��3��ʵ����֤���ٶ�����̼��ʹ����ʯ��ˮ����ǣ���������������һ��մ�г���ʯ��ˮ���ձ���ס���̣���Ŀ����Ϊ����֤����A��������ȼ��ʱ���ɵĶ�����̼��
�ڴ�������������һ��ɶ���IJ���Ƭ���ڰ����ϣ�����Ƭ��û��ˮ����˵�����̲���ˮ������
�۴�������������ȼ�ŵ�ľ��ȥ����̣���Ҫ�Ӵ���о���������������±���ȼ��˵�����̾��п�ȼ�ԣ����֤���˼���C����ȷ�ԣ�����Ϊˮ�����Ͷ�����̼������ȼ�գ�����ͬʱ֤���˼���AB�Dz���ȷ�Ľ⣺
��4�����������غ㶨�ɿ�֪����Ϊ�ڻ�ѧ��Ӧǰ���Ԫ�������������ȣ����Ը��л�����һ������̼���⣬��̼������Ӧ���ڶ�����̼��̼��������ˮ�������������˸��л����к���C������Ϊ��8.8g��
��100%=2.4g������H��������7.2g��
��100%=0.8g��2.4g+0.8g=3.2g��10g�����Ը��л�����һ��������Ԫ�أ�
�ʴ�Ϊ��
��1��abdeh��
��2��cfgij�������Ϊ3�㣻��������������
��3����A�� ��B�� ��C��AB��������̼��ˮ����������ȼ�գ�
��4����Ϊ�ڻ�ѧ��Ӧǰ���Ԫ�������������ȣ����Ը��л�����һ������̼���⣬��̼������Ӧ���ڶ�����̼��̼��������ˮ�������������˸��л����к���C������Ϊ��8.8g��
��100%=2.4g������H��������7.2g��
��100%=0.8g��2.4g+0.8g=3.2g��10g�����Ը��л�����һ��������Ԫ�أ�
��2���ص�Ӧ���ҵ�����ȼ�ճ��ֻ����һЩ��ѧ���ʣ�cfgij��������ȼ�չ����вű��ֳ��������ʣ�Ȼ����ʵ���Ҿƾ��ƻ����һЩ����дһЩ������û�е������磺�����Ϊ3�㣻��������������
��3��ʵ����֤���ٶ�����̼��ʹ����ʯ��ˮ����ǣ���������������һ��մ�г���ʯ��ˮ���ձ���ס���̣���Ŀ����Ϊ����֤����A��������ȼ��ʱ���ɵĶ�����̼��
�ڴ�������������һ��ɶ���IJ���Ƭ���ڰ����ϣ�����Ƭ��û��ˮ����˵�����̲���ˮ������
�۴�������������ȼ�ŵ�ľ��ȥ����̣���Ҫ�Ӵ���о���������������±���ȼ��˵�����̾��п�ȼ�ԣ����֤���˼���C����ȷ�ԣ�����Ϊˮ�����Ͷ�����̼������ȼ�գ�����ͬʱ֤���˼���AB�Dz���ȷ�Ľ⣺
��4�����������غ㶨�ɿ�֪����Ϊ�ڻ�ѧ��Ӧǰ���Ԫ�������������ȣ����Ը��л�����һ������̼���⣬��̼������Ӧ���ڶ�����̼��̼��������ˮ�������������˸��л����к���C������Ϊ��8.8g��
| 12 |
| 44 |
| 2 |
| 18 |
�ʴ�Ϊ��
��1��abdeh��
��2��cfgij�������Ϊ3�㣻��������������
��3����A�� ��B�� ��C��AB��������̼��ˮ����������ȼ�գ�
��4����Ϊ�ڻ�ѧ��Ӧǰ���Ԫ�������������ȣ����Ը��л�����һ������̼���⣬��̼������Ӧ���ڶ�����̼��̼��������ˮ�������������˸��л����к���C������Ϊ��8.8g��
| 12 |
| 44 |
| 2 |
| 18 |
���������⿼��֪ʶ��Ϊ�ۺϣ���ȫ�濼��������ȼ��ʱ��ʵ�������ֿ����˼�������֪ʶ��Ҫ��������ϸ�ķ������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ��ѧ��ȤС�����λͬѧ��������Ҫ�ɷ���ʯ��������ȼ�ս���������̽����
��ѧ��ȤС�����λͬѧ��������Ҫ�ɷ���ʯ��������ȼ�ս���������̽����
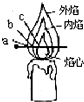 ��ѧ��ȤС�����λͬѧ��������Ҫ�ɷ���ʯ��������ȼ�ս���������̽����
��ѧ��ȤС�����λͬѧ��������Ҫ�ɷ���ʯ��������ȼ�ս���������̽����