��Ŀ����
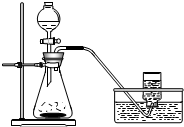 ������һ��Ӧ��ǰ���dz�����������Դ��������ȡ�������������������Ը����
������һ��Ӧ��ǰ���dz�����������Դ��������ȡ�������������������Ը������1��ʵ���ҿ���п��ϡ���ᷴӦ��ȡ������װ������ͼ��ʾ���仯ѧ����ʽΪ
��2��ͨ�����ˮ�ķ���Ҳ���Ի����������Ҫ���Ĵ����ĵ��ܣ���������Դ�������ù����в�����Ҫ����Ŀ�����
A��̽�������������ķ��� B����δ�������ȼ�յIJ��� C��������������������������⣮
���������ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ����д��ѧ����ʽ��ʵ�������ù�������ֽ���ȡ����ʱ����Ҫ���ȣ�����������������ˮ������ȼ�յ���������ˮ������Ⱦ������
����⣺��1��п��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ��Zn+H2SO4�TZnSO4+H2����
��װ�û�������ʵ������ȡ���ռ����������������
���������ڴ����������·ֽ�����ˮ�������Ļ�ѧ����ʽΪ��2H2O2
2H2O+O2����
��2������Դ�������ù�������Ҫ����Ŀ�����̽�������������ķ�����������������������������⣮����Ҫ����Ŀ�������δ�������ȼ�յIJ�����B��
��װ�û�������ʵ������ȡ���ռ����������������
���������ڴ����������·ֽ�����ˮ�������Ļ�ѧ����ʽΪ��2H2O2
| ||
��2������Դ�������ù�������Ҫ����Ŀ�����̽�������������ķ�����������������������������⣮����Ҫ����Ŀ�������δ�������ȼ�յIJ�����B��
�����������Ҫ���������غ㶨�ɵ����ݣ�ֻ������������ȷ����д��ѧ����ʽ��

��ϰ��ϵ�д�
 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�
�����Ŀ