��Ŀ����
ijУ��ȤС���ͬѧ���ۺ�ʵ����У���ˮ�೧����һ��ʯ��ʯ��Ʒ������Ա�������Ǵ���Ʒ�к��е������Ƕ������裬��������������ˮ�����������ᷴӦ��Ϊ�˲ⶨ��ʯ��ʯ�Ĵ��ȣ���ȤС���ͬѧȡ��8.00g����ʯ��ʯ��Ʒ����ʵ�������е�δ֪��������������ϡ����100�˷�5�μ��룬��ַ�Ӧ�����ˡ�����Ȳ�����������õ��������ݣ�| ʵ �� �� �� | 1 | 2 | 3 | 4 | 5 |
| ����ϡ���������/g | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| ʣ����������/g | 6.00 | m | 2.00 | 1.20 | 1.20 |
��l������m��ֵΪ______g��
��2����ʯ��ʯ��Ʒ�Ĵ��ȣ�
��3�����õ�ϡ���������ʵ�����������
��4����2��ʵ���������Һ�����ʵ�����������
���𰸡���������1����������ͬ���������ᷴӦ����ʯ��ʯ�������ԭ����з�����
��2�����ݱ������ݿɷ��������ʵ�����Ϊ1.2g��
��3���������ݿ�֪��ÿ����20g���ᣬ��Ӧ��̼��Ƶ�����Ϊ2.00g���ɸ��ݻ�ѧ����ʽ������������ʵ�������
��4���ɸ��ݻ�ѧ����ʽ�����������40g�������Ȼ��Ƶ��������ų��Ķ�����̼�����������������غ����������Һ������������������𰸣�
����⣺��1�����ݱ������ݿɵã�ÿ����20g���ᣬ������������2.00g������m��ֵΪ6.00-2.00g=4.00g
�ʴ�Ϊ��4.00g
��2���ɱ������ݿ�֪���Ĵκ͵���ι�����������ٱ仯���������ʵ�����Ϊ1.20g��
����ʯ��ʯ����������= ×100%=85.0%
×100%=85.0%
�𣺸�ʯ��ʯ��Ʒ�Ĵ���85.0%��
��3�������40g����ʱ����Ӧ��̼��Ƶ�����Ϊ4.00g
���ʱ�μӷ�Ӧ��HCl������Ϊx������CaCl2������Ϊy���ų�CO2���������Ϊz��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
4.00g x y z

��֮�ã�X=2.92g y=4.44g z=1.76g
�����������������=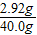 ×100%=7.30%
×100%=7.30%
�����õ�ϡ���������ʵ���������7.30%��
��4�����������غ㶨�ɿ�֪CaCl2��Һ������=40.0g+4g-1.76g=42.2g
���������Ȼ�����Һ����������=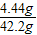 ×100%=10.5%
×100%=10.5%
�𣺵�2��ʵ���������Һ�����ʵ���������10.5%��
������������Ҫ�������û�ѧ����ʽ���м����������ͬʱҲ������ѧ���������ݣ��ҳ����ɵ�������
��2�����ݱ������ݿɷ��������ʵ�����Ϊ1.2g��
��3���������ݿ�֪��ÿ����20g���ᣬ��Ӧ��̼��Ƶ�����Ϊ2.00g���ɸ��ݻ�ѧ����ʽ������������ʵ�������
��4���ɸ��ݻ�ѧ����ʽ�����������40g�������Ȼ��Ƶ��������ų��Ķ�����̼�����������������غ����������Һ������������������𰸣�
����⣺��1�����ݱ������ݿɵã�ÿ����20g���ᣬ������������2.00g������m��ֵΪ6.00-2.00g=4.00g
�ʴ�Ϊ��4.00g
��2���ɱ������ݿ�֪���Ĵκ͵���ι�����������ٱ仯���������ʵ�����Ϊ1.20g��
����ʯ��ʯ����������=
 ×100%=85.0%
×100%=85.0%�𣺸�ʯ��ʯ��Ʒ�Ĵ���85.0%��
��3�������40g����ʱ����Ӧ��̼��Ƶ�����Ϊ4.00g
���ʱ�μӷ�Ӧ��HCl������Ϊx������CaCl2������Ϊy���ų�CO2���������Ϊz��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73 111 44
4.00g x y z

��֮�ã�X=2.92g y=4.44g z=1.76g
�����������������=
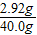 ×100%=7.30%
×100%=7.30% �����õ�ϡ���������ʵ���������7.30%��
��4�����������غ㶨�ɿ�֪CaCl2��Һ������=40.0g+4g-1.76g=42.2g
���������Ȼ�����Һ����������=
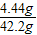 ×100%=10.5%
×100%=10.5% �𣺵�2��ʵ���������Һ�����ʵ���������10.5%��
������������Ҫ�������û�ѧ����ʽ���м����������ͬʱҲ������ѧ���������ݣ��ҳ����ɵ�������

��ϰ��ϵ�д�
�����Ŀ




 �ڽ��껯ѧ����У�ijУ��ȤС���ͬѧ����ʦָ��������������ѧʵ�飬�Իش�������⣮
�ڽ��껯ѧ����У�ijУ��ȤС���ͬѧ����ʦָ��������������ѧʵ�飬�Իش�������⣮
 Ũ����
Ũ����