��Ŀ����
����Ŀ����ҵ��ʯ��ʯ�����շ����еĶ���������ȡʯ�ࣨ��Ҫ�ɷ�Ϊ����ƣ�������������ͼ����Ҫ�ķ�ӦΪ��2CaCO3+2SO2+O2�T2CaSO4+2X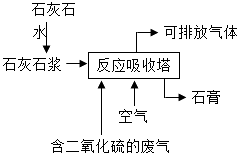
��1��������ʯ��ʯ��ˮ�Ƴ�ʯ��ʯ����Ŀ���� �� ��Ӧ��X�Ļ�ѧʽΪ �� ��Ԫ�صĻ��ϼ��ڷ�Ӧǰ��ı仯Ϊ ��
��2�����ٶ���������ŷţ���ҪΪ��������ţ�
A.��������ЧӦ
B.���������γ�
C.��ֹ�ƻ�������
��3����������������2000�ַ����е�SO2 �� ������Ҫ��5��̼��Ƶ�ʯ��ʯ����������SO2������������
���𰸡�
��1���������ն�������CO2����+4��Ϊ+6
��2��B
��3��
������ж������������Ϊx��
��2CaCO3+2SO2+O2![]() 2CaSO4+2CO2��
2CaSO4+2CO2��
200 128
5t x
![]()
x=3.2t
������SO2����������=![]()
![]() =0.16%
=0.16%
�𰸣�������SO2����������Ϊ0.16%��
���������⣺��1��������ʯ��ʯ��ˮ�Ƴ�ʯ��ʯ����Ŀ���DZ������ն�������
����2CaCO3+2SO2+O2�T2CaSO4+2x��֪�����������غ㶨�ɿ�֪ÿ��x�к���1��̼ԭ�Ӻ�2����ԭ�ӣ�����x�Ƕ�����̼�����CO2��
��Ԫ�صĻ��ϼ��ڷ�ӦǰΪ+4����Ӧ��Ϊ+6����Ӧǰ��ı仯Ϊ��+4��Ϊ+6����2��
A������ЧӦ��Ҫ�Ƕ�����̼�����������ŷŵ��������γɵģ������������ŷ��أ���ѡ�����
B��ú��ȼ��ȼ�����ɶ�������������������ˮ���γ��������Ҫԭ�������������ŷ��йأ���ѡ����ȷ��
C���������ƻ��Ǻ���������ŷŵ���������ɵģ������������ŷ��أ���ѡ�����
3��������ж������������Ϊx��
��2CaCO3+2SO2+O2![]() 2CaSO4+2CO2 ��
2CaSO4+2CO2 ��
200 128
5t x![]()
x=3.2t
������SO2����������=![]()
![]() =0.16%
=0.16%
�𰸣�������SO2����������Ϊ0.16%��
�𰸣���1���������ն�������CO2 �� ��+4��Ϊ+6����2��B����3��������SO2����������Ϊ0.16%��
�����㾫����������Ĺؼ��������������غ㶨�ɼ���Ӧ�õ����֪ʶ�����բ������غ㶨��ֻ�����ڻ�ѧ�仯���������������仯���ڲ��μӷ�Ӧ����������������������������������ܼ��롰�ܺ͡��У���Ҫ���ǿ����е������Ƿ�μӷ�Ӧ�����ʣ������壩������©���Լ��Ը��ݻ�ѧ��Ӧ����ʽ�ļ�������⣬�˽�����ʼ�������=ϵ������Է�������֮�ȣ�